Phosphorylation of actin-depolymerizing factor/cofilin by LIM-kinase mediates amyloid beta-induced degeneration: a potential mechanism of neuronal dystrophy in Alzheimer's disease - PubMed (original) (raw)
Comparative Study
Phosphorylation of actin-depolymerizing factor/cofilin by LIM-kinase mediates amyloid beta-induced degeneration: a potential mechanism of neuronal dystrophy in Alzheimer's disease
Lorena Heredia et al. J Neurosci. 2006.
Abstract
Deposition of fibrillar amyloid beta (fAbeta) plays a critical role in Alzheimer's disease (AD). We have shown recently that fAbeta-induced dystrophy requires the activation of focal adhesion proteins and the formation of aberrant focal adhesion structures, suggesting the activation of a mechanism of maladaptative plasticity in AD. Focal adhesions are actin-based structures that provide a structural link between the extracellular matrix and the cytoskeleton. To gain additional insight in the molecular mechanism of neuronal degeneration in AD, here we explored the involvement of LIM kinase 1 (LIMK1), actin-depolymerizing factor (ADF), and cofilin in Abeta-induced dystrophy. ADF/cofilin are actin-binding proteins that play a central role in actin filament dynamics, and LIMK1 is the kinase that phosphorylates and thereby inhibits ADF/cofilin. Our data indicate that treatment of hippocampal neurons with fAbeta increases the level of Ser3-phosphorylated ADF/cofilin and Thr508-phosphorylated LIMK1 (P-LIMK1), accompanied by a dramatic remodeling of actin filaments, neuritic dystrophy, and neuronal cell death. A synthetic peptide, S3 peptide, which acts as a specific competitor for ADF/cofilin phosphorylation by LIMK1, inhibited fAbeta-induced ADF/cofilin phosphorylation, preventing actin filament remodeling and neuronal degeneration, indicating the involvement of LIMK1 in Abeta-induced neuronal degeneration in vitro. Immunofluorescence analysis of AD brain showed a significant increase in the number of P-LIMK1-positive neurons in areas affected with AD pathology. P-LIMK1-positive neurons also showed early signs of AD pathology, such as intracellular Abeta and pretangle phosphorylated tau. Thus, LIMK1 activation may play a key role in AD pathology.
Figures
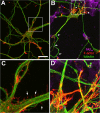
Figure 1.
fAβ induces neuronal dystrophy associated with altered organization of actin filaments. Rat hippocampal cultures were treated with vehicle or 20 μ
m
fAβ1–40 (fAβ40) at day 7, fixed 1 d later, and stained with rhodamine–phalloidin (red) and with antibodies to Aβ (blue) and to tubulin class III (green). A, B, Confocal images taken at 40× magnification. C, D, Enlargements of the area depicted in A and B, respectively. A, C, Control neurons exhibit smooth and healthy appearance of neurites with small bundles of actin filaments at the tips of neurites and in delicate filopodia extending from the neuritic shaft (C, arrows). B, Neurons treated with fAβ show aberrant neuritic morphology, including altered neuritic caliber, increased tortuosity (arrows), reduced extension of the neuritic network, and aberrant branching and sprouting of neurites. D, Large bundles of actin filaments are observed in dystrophic neurites in proximities to fAβ deposits (B, arrowhead). Scale bar, 20 μm.
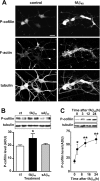
Figure 2.
fAβ induces neuronal dystrophy and increased phosphorylation of cofilin. A, Rat hippocampal cultures were treated at day 7 with vehicle (control) or 20 μ
m
fibrillar Aβ1–40 (fAβ40) and fixed at day 8. Confocal images were taken of cultures stained with rhodamine–phalloidin (F-actin) and with antibodies to P-cofilin and to tubulin class III (tubulin). Note that fAβ-treated cultures exhibit an increased level in P-cofilin and altered distribution of F-actin and significant neuritic dystrophy. Scale bar, 20 μm. B, Hippocampal cultures were treated with vehicle (ct), 20 μ
m
fibrillar Aβ1–40 (fAβ40), or 20 μ
m
soluble Aβ1–40 (sAβ40) and harvested after 24 h. Cell lysates were analyzed in triplicate by Western blot with antibodies against P-cofilin and tubulin; densitometric determination showed a significant increase in the P-cofilin level after fAβ treatment (∗p < 0.016, ANOVA followed by the LSD post hoc test). Similar results were obtained in at least three independent experiments. C, Rat hippocampal cultures were treated with 20 μ
m
fAβ25–35 (fAβ35) for the indicated period of time, and the levels of P-cofilin and tubulin were analyzed in triplicate samples by densitometry after Western blot with specific antibodies. A significant increase in the P-cofilin level was observed after fAβ at all time points analyzed (∗p < 0.005, ∗∗p < 0.001, ANOVA followed by the LSD post hoc test). Plots are shown in arbitrary desitometric units (ADU). Error bars indicate SEM.
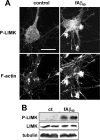
Figure 3.
Activation of LIMK1 by fAβ. Rat hippocampal cultures were treated with vehicle (control) or 20 μ
m
fAβ1–40 (fAβ40) from day 7 to day 8 and fixed for immunolabeling or harvested for Western blot analysis and labeled with antibody to LIMK1 and P-LIMK1. A, Hippocampal neurons were immunolabeled with anti-P-LIMK1 and stained with rhodamine–phalloidin (F-actin), and immunofluorescent images were taken with identical settings; note the increase in P-LIMK1 after fAβ1–40 treatment, which mostly overlap with increased F-actin. Scale bar, 20 μm. B, Western blot showing the levels of P-LIMK1, LIMK1, and tubulin in control (ct) and cultures treated with fAβ1–40.
Figure 4.
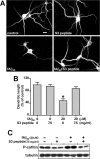
S3 peptide inhibits fAβ-induced phosphorylation of cofilin by LIMK1. A, Structure of S3 peptide. B, Time- and concentration-dependent inhibition of endogenous cofilin phosphorylation by S3 peptide. Rat hippocampal cultures were treated with vehicle or with 20 μg/ml S3 peptide for the indicated time period (left) or treated with the indicated concentration of S3 peptide for 4 h (right); the level of P-cofilin and tubulin were analyzed by Western blot. C, Rat hippocampal cultures were treated with the indicated concentrations of S3 peptide and fAβ1–40 (fAβ40) for 6 h, and the level of P-cofilin was analyzed in triplicate samples by Western blot and densitometry. Plots are shown in arbitrary desitometric units (ADU). ∗∗p < 0.0003, fAβ versus control and S3 peptide (by ANOVA). Note that treatment with S3 peptide inhibits the increase in P-cofilin induced by fAβ. Error bars indicate SEM. D, Rat hippocampal cultures were treated for 6 h with vehicle (control), 20 μg/ml S3 peptide, or 20 μ
m
fAβ1–40, fixed, and immunolabeled with anti-P-cofilin antibody and F-actin. Confocal microscopy images were taken with identical settings. Scale bar, 20 μm.
Figure 5.
S3 peptide prevents fAβ-induced neuritic loss. Rat hippocampal cultures were treated for 24 h with vehicle (control) and 20 μ
m
fAβ1–40 (fAβ40) with or without 7.5 μg/ml S3 peptide. The cultures were fixed and immunolabeled with anti-tubulin class III antibody and analyzed by immunofluorescence microscopy. Fluorescent images were taken at 40× magnification with identical settings. Note that S3 peptide dramatically reduces the loss of neurites induced by fAβ. Scale bar, 20 μm.
Figure 6.
S3 peptide counteracts fAβ-induced dendritic retraction. Rat hippocampal cultures were treated for 24 h with vehicle (control) or 20 μ
m
fAβ1–40 (fAβ40), with or without 7.5 μg/ml S3 peptide, and fixed and immunolabeled with anti-MAP2 antibody. A, Fluorescent images were taken at 40× magnification. Note that S3 peptide prevents the reduction in dendritic length induced by fAβ. Scale bar, 20 μm. B, The length of MAP2-positive dendrites was determined in 80 neurons from triplicate samples using MetaMorph software; statistical comparisons were made by ANOVA and the LSD post hoc test. ∗p < 0.0001, fAβ versus control and S3 peptide-treated cultures. Error bars indicate SEM. C, Western blot analysis showing the levels of P-cofilin and tubulin after 24 h of treatment with the indicated concentrations of fAβ1–40 and S3 peptide; samples are shown by duplicate.
Figure 7.
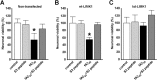
LIMK1 and fAβ-induced neuronal death. Rat hippocampal cultures were treated with vehicle (control) or 20 μ
m
fAβ1–40 (fAβ40), with or without 7.5 μg/ml S3 peptide, and the number of viable neurons was scored. A, In nontransfected cultures, the effect of fAβ1–40 and S3 peptide on neuronal viability was assessed 24 h after treatment by Hoechst staining. B, C, In cultures that were cotransfected with GFP and either wt-LIMK1 (B) or kd-LIMK1 (C), neuronal viability was assessed 18 h after treatment by scoring GFP-expressing neurons. A, Representative experiment showing the effect of fAβ and S3 peptide in neuronal viability. More than 900 neurons were scored, and similar results were found in three independent experiments. Statistical comparisons were made by ANOVA (F(3,36) = 23.32) and the LSD post hoc test. ∗p < 0.001, fAβ versus control, S3 peptide, and fAβ+S3peptide. **B_**, Effect of fAβ and S3 peptide on neuronal viability in GFP/wt-LIMK1-cotransfected neurons. A representative experiment is shown, >170 neurons were scored, and similar results were found in three independent experiments. Statistical comparisons were made by ANOVA (F(3,12) = 4.1464) and the LSD post hoc test. ∗_p < 0.01, fAβ versus control, S3 peptide, and fAβ+S3peptide. Note that toxicity of fAβ is blocked by S3 peptide in nontransfected neuronal cultures that express endogenous levels of LIMK1 and in cultures that were transfected with wt-LIMK1. **_C_**, Effect of fAβ and S3 peptide on neuronal viability in GFP/kd-LIMK1-cotransfected culture. A representative experiment is shown, >110 neurons were scored, and similar results were found in three independent experiments. No significant effect of fAβ in neuronal viability was observed; statistical comparisons were made by ANOVA (F(3,12) = 0.69154; p = NS). Note that kd-LIMK transfection inhibits fAβ-induced neuronal death. Error bars indicate SEM.
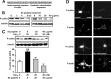
Figure 8.
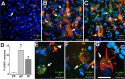
P-LIMK immunolabeling is associated with degenerative markers in sporadic human AD brains. A–C, E–G, Double immunofluorescence for P-LIMK1 and Aβ or phosphorylated tau in AD brains. Increased neuronal P-LIMK expression (1:50 anti-P-LIMK; green fluorescence) in cortical region exhibiting abundant AD pathology [1:100 anti-Aβ clone 6E10, red fluorescence (A, B, E), or 1:400 anti-hyperphosphorylated tau (PHF-1), red fluorescence (C, F, G)]. Cellular nuclei were counterstained with Hoechst (blue fluorescence). A–C, Lower magnification illustrates minimal P-LIMK expression in cortical regions free of AD lesions (A, arrow) and intense P-LIMK expression in neurons close to regions showing amyloid plaques and tangles (B, C, arrows, respectively). D, The number of P-LIMK1-positive neurons in AD brain was assessed on conventional fluorescence acquisition images in areas of the entorhinal cortex without AD pathology (PF) and in adjacent areas of the same cortex showing Aβ pathology (AP) or tau pathology (TP). Statistical comparison was made by Student's t test (∗p < 0.0001 vs PF). E, Higher-magnification images revealed P-LIMK localization in neurons with detectable intracellular Aβ42 labeling, in the proximities of an amyloid plaque. F–G, P-LIMK is associated with neurons displaying different degrees of tau pathology (arrows), which are localized near mature neurofibrillary tangles (F, arrowhead). Images in A–C are conventional fluorescence acquisitions, and images in E–G are rendered images of 10 optical sections; each stack rendered was 3.75 μm thick. _Z_-stacks were generated and processed with the Apotome device (Zeiss). Scale bars, 20 μm.
Similar articles
- Cofilin, a Master Node Regulating Cytoskeletal Pathogenesis in Alzheimer's Disease.
Kang DE, Woo JA. Kang DE, et al. J Alzheimers Dis. 2019;72(s1):S131-S144. doi: 10.3233/JAD-190585. J Alzheimers Dis. 2019. PMID: 31594228 Free PMC article. Review. - C-terminal fragments of amyloid precursor proteins increase cofilin phosphorylation by LIM kinase in cultured rat primary neurons.
Cheng L, Chen H, Li C, Xu C, Xu YJ. Cheng L, et al. Neuroreport. 2019 Jan 2;30(1):38-45. doi: 10.1097/WNR.0000000000001162. Neuroreport. 2019. PMID: 30444792 - Fibrillar amyloid-β1-42 modifies actin organization affecting the cofilin phosphorylation state: a role for Rac1/cdc42 effector proteins and the slingshot phosphatase.
Mendoza-Naranjo A, Contreras-Vallejos E, Henriquez DR, Otth C, Bamburg JR, Maccioni RB, Gonzalez-Billault C. Mendoza-Naranjo A, et al. J Alzheimers Dis. 2012;29(1):63-77. doi: 10.3233/JAD-2012-101575. J Alzheimers Dis. 2012. PMID: 22204905 - Activated actin-depolymerizing factor/cofilin sequesters phosphorylated microtubule-associated protein during the assembly of alzheimer-like neuritic cytoskeletal striations.
Whiteman IT, Gervasio OL, Cullen KM, Guillemin GJ, Jeong EV, Witting PK, Antao ST, Minamide LS, Bamburg JR, Goldsbury C. Whiteman IT, et al. J Neurosci. 2009 Oct 14;29(41):12994-3005. doi: 10.1523/JNEUROSCI.3531-09.2009. J Neurosci. 2009. PMID: 19828813 Free PMC article. - Cofilin-mediated neurodegeneration in Alzheimer's disease and other amyloidopathies.
Maloney MT, Bamburg JR. Maloney MT, et al. Mol Neurobiol. 2007 Feb;35(1):21-44. doi: 10.1007/BF02700622. Mol Neurobiol. 2007. PMID: 17519504 Review.
Cited by
- Morphological and molecular changes in aging rat prelimbic prefrontal cortical synapses.
Bloss EB, Puri R, Yuk F, Punsoni M, Hara Y, Janssen WG, McEwen BS, Morrison JH. Bloss EB, et al. Neurobiol Aging. 2013 Jan;34(1):200-10. doi: 10.1016/j.neurobiolaging.2012.05.014. Epub 2012 Jun 22. Neurobiol Aging. 2013. PMID: 22727942 Free PMC article. - Extracellular Pgk1 enhances neurite outgrowth of motoneurons through Nogo66/NgR-independent targeting of NogoA.
Lin CY, Wu CL, Lee KZ, Chen YJ, Zhang PH, Chang CY, Harn HJ, Lin SZ, Tsai HJ. Lin CY, et al. Elife. 2019 Jul 30;8:e49175. doi: 10.7554/eLife.49175. Elife. 2019. PMID: 31361595 Free PMC article. - Cofilin, a Master Node Regulating Cytoskeletal Pathogenesis in Alzheimer's Disease.
Kang DE, Woo JA. Kang DE, et al. J Alzheimers Dis. 2019;72(s1):S131-S144. doi: 10.3233/JAD-190585. J Alzheimers Dis. 2019. PMID: 31594228 Free PMC article. Review. - Inhibition of Rac1 in ventral hippocampal excitatory neurons improves social recognition memory and synaptic plasticity.
Zhang H, Ben Zablah Y, Zhang H, Liu A, Gugustea R, Lee D, Luo X, Meng Y, Li S, Zhou C, Xin T, Jia Z. Zhang H, et al. Front Aging Neurosci. 2022 Jul 22;14:914491. doi: 10.3389/fnagi.2022.914491. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35936771 Free PMC article. - ICP4-Associated Activation of Rap1b Facilitates Herpes Simplex Virus Type I (HSV-1) Infection in Human Corneal Epithelial Cells.
Zhang B, Ding J, Ma Z. Zhang B, et al. Viruses. 2023 Jun 27;15(7):1457. doi: 10.3390/v15071457. Viruses. 2023. PMID: 37515145 Free PMC article.
References
- Aizawa H, Wakatsukim S, Ishii A, Moriyama K, Sasaki Y, Ohashi K, Sekine-Aizawa Y, Sehara-Fujisawa A, Mizuno K, Goshima Y, Yahara I (2001). Phosphorylation of cofilin by LIMkinase is necessary for semaphorin 3A-induced growth cone collapse. Nat Neurosci 4:367–373. - PubMed
- Arber S, Barbayannis F, Hanser H, Schneider C, Stanyon C, Bernard O, Caroni P (1998). Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature 393:805–812. - PubMed
- Arrasate M, Mitra S, Schweitzer E, Segal M, Finkbeiner S (2004). Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature 431:810. - PubMed
- Bamburg JR, Wiggan OP (2002). ADF/cofilin and actin dynamics in disease. Trends Cell Biol 12:598–605. - PubMed
- Benson DL, Schnapp LM, Shapiro L, Huntley GW (2000). Making memories stick: cell-adhesion molecules in synaptic plasticity. Trends Cell Biol 10:473–482. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials