Enhanced sensitivity of Kv channels to hypoxia in the rabbit carotid body in heart failure: role of angiotensin II - PubMed (original) (raw)
Comparative Study
Enhanced sensitivity of Kv channels to hypoxia in the rabbit carotid body in heart failure: role of angiotensin II
Yu-Long Li et al. J Physiol. 2006.
Abstract
Angiotensin II (Ang II) plays an important role in the enhanced chemoreflex function that occurs in congestive heart failure (CHF), but the mechanism of this effect within the carotid body (CB) is not known. We investigated the sensitivity of Ca2+-independent, voltage-gated K+ (Kv) channels to hypoxia in CB glomus cells from CHF rabbits, and whether endogenous angiotensin II (Ang II) modulates this action. Using the conventional whole-cell patch clamp technique, we found that Kv currents (IK) under normoxic conditions were blunted in the CB glomus cells from CHF rabbits compared with sham rabbits. In addition, the inhibition of IK and the decrease of resting membrane potential (RMP) induced by hypoxia were greater in CHF versus sham glomus cells. Ang II, at 100 pM, had no direct effect on IK at constant normoxic PO2, but increased the sensitivity of IK and RMP to hypoxia in sham glomus cells. In CHF glomus cells, an AT1 receptor (AT1R) antagonist, L-158 809 (1 microM), alone did not affect IK at normoxia, but it decreased the sensitivity of IK and RMP to hypoxia. At higher concentrations, Ang II dose dependently (0.1-100 nM) reduced IK under constant normoxic conditions in sham and CHF glomus cells, with threshold concentrations of about 900 and 600 pM, respectively. Immunocytochemical and Western blot assessments demonstrated the down-expression of Kv3.4 but not Kv4.3 channels in CHF glomus cells. These results indicate that: (1) Ang II/AT1R signalling increases the sensitivity of Kv channels to hypoxia in CB glomus cells from CHF rabbits; (2) high concentrations of Ang II (> 1 nM) directly inhibit IK in CB glomus cells from sham and CHF rabbits; (3) changes in Kv channel protein expression (Kv3.4 versus Kv4.3) in the CB glomus cell may contribute to the suppression of IK and enhanced sensitivity of IK to hypoxia in CHF.
Figures
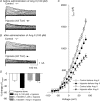
Figure 1. Effect of Ang II on _I_K in CB glomus cells from sham and CHF rabbits under normoxic conditions (_P_O2 = 104 ± 1.4 Torr)
_I_K was evoked by 400 ms depolarizing pulses from −80 to +80 mV, 10 mV steps). A and _B, I_K from a sham and CHF glomus cell, respectively, before (control) and after treatment with 100 n
m
Ang II in the extracellular medium. C, peak I_–_V relationships (n = 10 cells from 7 rabbits in each group) from sham and CHF glomus cells obtained before and after treatment with 100 n
m
Ang II. Data are means ±
s.e.m.
*P < 0.05 versus sham-control; #P < 0.05 versus CHF-control. D, dose–response curves of extracellular Ang II concentration versus percentage reduction of peak _I_K, in sham and CHF glomus cells. Data are means ±
s.e.m.
n = 10 cells from 7 rabbits for each point. *P < 0.05 versus sham. E, peak _I_K of sham and CHF glomus cells before (control) and after exposure to either 100 n
m
Ang II, 1 μ
m
L-158, 809 (AT1R antagonist), or 100 n
m
Ang II + 1 μ
m
L-158, 809. Data are means ±
s.e.m.
n = 10 cells from 7 rabbits in each group. *P < 0.05 versus control; #P < 0.05 versus Ang II; †P < 0.05 versus sham). Peak _I_K in D and E measured in response to a test pulse from −80 to +70 mV.
Figure 2. Effects of Ang II and L-158, 809 on the sensitivity of _I_K to hypoxia (_P_O2 = 41.7 ± 2.2 Torr) in CB glomus cells from sham rabbits
_I_K was elicited as in Fig. 1. A and B, effects of hypoxia on _I_K before (A) and after (B) administration of 100 p
m
Ang II to the extracellular medium. C, peak I_–_V relationships in 10 cells from 7 sham rabbits for data illustrated in A and B. Data are means ±
s.e.m.
*P < 0.05 versus control; #P < 0.05 versus hypoxia before Ang II. D, percentage change of _I_K by hypoxia (_I_control–_I_hypoxia)/_I_control) in sham glomus cells before (hypoxia alone) and after exposure to either 100 p
m
Ang II, 1 μ
m
L-158, 809 (AT1R antagonist), or 100 p
m
Ang II + 1 μ
m
L-158, 809. Data are means ±
s.e.m.
n = 10 cells from 7 rabbits in each condition. *P < 0.05 versus hypoxia alone; #P < 0.05 versus Ang II (100 p
m
) + hypoxia)
Figure 3. Effects of Ang II and L-158, 809 on the sensitivity of _I_K to hypoxia (_P_O2 = 42.2 ± 1.3 Torr) in CB glomus cells from CHF rabbits
_I_K was elicited as in Fig. 1. A and B, effects of hypoxia on _I_K before (A) and after (B) administration of 100 p
m
Ang II to the extracellular medium. C, peak I_–_V relationships in 10 cells from 7 CHF rabbits for data illustrated in A and B. Data are means ±
s.e.m.
*P < 0.05 versus control; #P < 0.05 versus hypoxia before L-158, 809). D, percentage change of _I_K by hypoxia (_I_control–_I_hypoxia)/_I_control) in CHF glomus cells before (hypoxia alone) and after exposure to either 100 p
m
Ang II, 1 μ
m
L-158, 809 (AT1R antagonist), or 100 p
m
Ang II + 1 μ
m
L-158,809. Data are means ±
s.e.m.
n = 10 cells from 7 rabbits in each condition. *P < 0.05 versus hypoxia alone; #P < 0.05 versus Ang II (100 p
m
) + hypoxia).
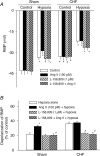
Figure 4. Effects of Hyproxia, Ang II, and L-158, 809 on RMP in CB glomus cells from sham and CHF rabbits
A, effect of hypoxia (41.9 ± 2.1 Torr) on resting membrane potential (RMP) at different conditions in CB glomus cells from sham and CHF rabbits. Data are means ±
s.e.m.
n = 8 cells from 5 rabbits in each condition. *P < 0.05 versus sham-normoxia or CHF-normoxia; #P < 0.05 versus sham-normoxia. B, percentage reduction of RMP by hypoxia (_I_control–_I_hypoxia)/_I_control) in sham and CHF glomus cells before (hypoxia alone) and after exposure to either 100 p
m
Ang II, 1 μ
m
L-158, 809 (AT1R antagonist), or 100 p
m
Ang II + 1 μ
m
L-158, 809. Data are means ±
s.e.m.
n = 8 cells from 5 rabbits in each condition. *P < 0.05 versus hypoxia alone; #P < 0.05 versus Ang II (100 p
m
) + hypoxia).
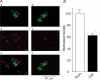
Figure 5. Co-localization of tyrosine hydroxylase (TH) and Kv3.4 in CB glomus cells from sham and CHF rabbits
A, CB from a sham rabbit (a_–_c) and CHF rabbit (d_–_f). Green immunofluorescent image for TH in a and d, red immunofluorescent image for Kv3.4 showed in b and e, and the merged image for overlap of TH and Kv3.4 in c and f. B, normalized fluorescence intensity for Kv3.4 in CB glomus cells from sham and CHF rabbits (see detail in Methods). Data are means ±
s.e.m.
n = 5 rabbits for each group. *P < 0.05 versus sham.
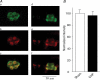
Figure 6. Co-localization of tyrosine hydroxylase (TH) and Kv4.3 in CB glomus cells from sham and CHF rabbits
A, CB from a sham (a_–_c) and CHF (d_–_f) rabbit. Green immunofluorescent image for TH in a and d, red immunofluorescent image for Kv4.3 in b and e, and the merged image for overlap of TH and Kv4.3 in c and f. B, normalized fluorescence intensity for Kv4.3 in CB glomus cells from sham and CHF rabbits (see detail in Methods). Data are means ±
s.e.m.
n = 5 rabbits for each group. *P < 0.05 versus sham.
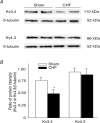
Figure 7. Protein expression of Kv3.4 and Kv4.3
The representative (A) and summary (B) data for protein expression of Kv3.4 and Kv4.3 in the CBs from sham and CHF rabbits. Data are means ±
s.e.m.
n = 4 samples in each group. *P < 0.05 versus sham.
Comment in
- Novel roles of a local angiotensin-generating system in the carotid body.
Leung PS. Leung PS. J Physiol. 2006 Aug 15;575(Pt 1):4. doi: 10.1113/jphysiol.2006.115550. Epub 2006 Jun 29. J Physiol. 2006. PMID: 16809356 Free PMC article. No abstract available.
Similar articles
- Angiotensin II enhances carotid body chemoreflex control of sympathetic outflow in chronic heart failure rabbits.
Li YL, Xia XH, Zheng H, Gao L, Li YF, Liu D, Patel KP, Wang W, Schultz HD. Li YL, et al. Cardiovasc Res. 2006 Jul 1;71(1):129-38. doi: 10.1016/j.cardiores.2006.03.017. Epub 2006 Mar 24. Cardiovasc Res. 2006. PMID: 16650840 - NADPH oxidase-derived superoxide anion mediates angiotensin II-enhanced carotid body chemoreceptor sensitivity in heart failure rabbits.
Li YL, Gao L, Zucker IH, Schultz HD. Li YL, et al. Cardiovasc Res. 2007 Aug 1;75(3):546-54. doi: 10.1016/j.cardiores.2007.04.006. Epub 2007 Apr 19. Cardiovasc Res. 2007. PMID: 17499230 Free PMC article. - Role of blood flow in carotid body chemoreflex function in heart failure.
Ding Y, Li YL, Schultz HD. Ding Y, et al. J Physiol. 2011 Jan 1;589(Pt 1):245-58. doi: 10.1113/jphysiol.2010.200584. Epub 2010 Nov 15. J Physiol. 2011. PMID: 21078591 Free PMC article. - Carotid body function in heart failure.
Schultz HD, Li YL. Schultz HD, et al. Respir Physiol Neurobiol. 2007 Jul 1;157(1):171-85. doi: 10.1016/j.resp.2007.02.011. Epub 2007 Feb 16. Respir Physiol Neurobiol. 2007. PMID: 17374517 Free PMC article. Review. - NO modulation of carotid body chemoreception in health and disease.
Moya EA, Alcayaga J, Iturriaga R. Moya EA, et al. Respir Physiol Neurobiol. 2012 Nov 15;184(2):158-64. doi: 10.1016/j.resp.2012.03.019. Epub 2012 Apr 5. Respir Physiol Neurobiol. 2012. PMID: 22516266 Review.
Cited by
- Relevance of the Carotid Body Chemoreflex in the Progression of Heart Failure.
Andrade DC, Lucero C, Toledo C, Madrid C, Marcus NJ, Schultz HD, Del Rio R. Andrade DC, et al. Biomed Res Int. 2015;2015:467597. doi: 10.1155/2015/467597. Epub 2015 Dec 8. Biomed Res Int. 2015. PMID: 26779536 Free PMC article. Review. - Mitochondria: In the Cross Fire of SARS-CoV-2 and Immunity.
Burtscher J, Cappellano G, Omori A, Koshiba T, Millet GP. Burtscher J, et al. iScience. 2020 Oct 23;23(10):101631. doi: 10.1016/j.isci.2020.101631. Epub 2020 Sep 29. iScience. 2020. PMID: 33015593 Free PMC article. Review. - Chemoreceptor hypersensitivity, sympathetic excitation, and overexpression of ASIC and TASK channels before the onset of hypertension in SHR.
Tan ZY, Lu Y, Whiteis CA, Simms AE, Paton JF, Chapleau MW, Abboud FM. Tan ZY, et al. Circ Res. 2010 Feb 19;106(3):536-45. doi: 10.1161/CIRCRESAHA.109.206946. Epub 2009 Dec 17. Circ Res. 2010. PMID: 20019330 Free PMC article. - The role of local renin-angiotensin system in arterial chemoreceptors in sleep-breathing disorders.
Fung ML. Fung ML. Front Physiol. 2014 Sep 5;5:336. doi: 10.3389/fphys.2014.00336. eCollection 2014. Front Physiol. 2014. PMID: 25249981 Free PMC article. Review. - A possible role for systemic hypoxia in the reactive component of pulmonary hypertension in heart failure.
Taylor BJ, Mojica CR, Olson TP, Woods PR, Frantz RP, Johnson BD. Taylor BJ, et al. J Card Fail. 2013 Jan;19(1):50-9. doi: 10.1016/j.cardfail.2012.11.005. J Card Fail. 2013. PMID: 23273594 Free PMC article.
References
- Brooks VL. Interactions between angiotensin II and the sympathetic nervous system in the long-term control of arterial pressure. Clin Exp Pharmacol Physiol. 1997;24:83–90. - PubMed
- Brooks VL, Osborn JW. Hormonal–sympathetic interactions in long-term regulation of arterial pressure: an hypothesis. Am J Physiol. 1995;268:R1343–R1358. - PubMed
- Cardin S, Li D, Thorin-Trescases N, Leung TK, Thorin E, Nattel S. Evolution of the atrial fibrillation substrate in experimental congestive heart failure: angiotensin-dependent and -independent pathways. Cardiovasc Res. 2003;60:315–325. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous