The guinea pig as a transmission model for human influenza viruses - PubMed (original) (raw)
The guinea pig as a transmission model for human influenza viruses
Anice C Lowen et al. Proc Natl Acad Sci U S A. 2006.
Abstract
The severity of epidemic and pandemic influenza outbreaks is dictated in part by the efficiency with which the causative strain transmits between human hosts. The mechanisms underlying influenza virus spread are poorly understood, in part because of the lack of a convenient animal model to study this phenomenon. Indeed, despite extremely efficient transmission among humans and virulence in the mouse model, we have shown that even the 1918 pandemic influenza virus does not transmit between mice. We therefore evaluated the guinea pig as a model mammalian host for influenza virus. Using the recent human isolate A/Panama/2007/99 (Pan/99) (H3N2) virus, we found that guinea pigs were highly susceptible to infection with the unadapted virus (ID(50) = 5 plaque-forming units). Pan/99 virus grew to high titers in the upper respiratory tract and was shed in nasal washings of infected animals. Moreover, influenza virus was transmitted from infected guinea pigs to noninfected guinea pigs housed in the same cage, an adjacent cage, and a cage placed 91 cm away. Our results demonstrate that influenza virus can pass between guinea pigs by means of droplet spread and thereby establish the suitability of the guinea pig as a model host for influenza virus transmission studies.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
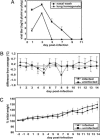
Fig. 1.
Characteristics of Pan/99 virus infection in guinea pigs. Fourteen guinea pigs were infected intranasally with 103 pfu of Pan/99 virus. The number of infected animals evaluated on each day varied as follows: day 1, n = 14; days 2 and 3, n = 12; days 4 and 5, n = 10; days 6 and 7, n = 8; days 8 and 9, n = 6; days 10–14, n = 4. At all time points, two mock-infected animals were assessed. (A) Viral titers in nasal washings and lung homogenates. At the indicated time points, nasal washings were collected from all guinea pigs, and two animals were killed and their lungs were removed. Infectious content of nasal wash samples and lung homogenates were then determined by plaque assay (limit of detection was 10 pfu/ml or 30 pfu/g). Nasal wash titers are expressed in pfu/ml, and lung titers are expressed in pfu/g of lung. (B) Change in body temperature over the course of infection. Temperatures were measured twice daily from 2 days before infection to 14 days p.i. The average preinfection temperature for each animal was subtracted from each p.i. temperature to obtain the change in body temperature for each guinea pig; the average change in temperature for all guinea pigs was plotted. (C) Change in body weight over the course of infection. Body weight was assessed daily and is expressed as the average percentage change in body weight for each animal.
Fig. 2.
Transmission of Pan/99 virus from intranasally infected guinea pigs to uninfected contacts. Three animals were inoculated with 102 pfu of Pan/99 virus. At 24 h p.i., each of these animals was placed in a cage (designated 1, 2, or 3) with one uninfected guinea pig. Nasal washes were performed at 48-h intervals, starting from 48 h p.i. for the inoculated animals (24 h p.c.).
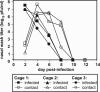
Fig. 3.
Droplet transmission of Pan/99 virus among guinea pigs. Representative results from two independent experiments are shown. Inoculated guinea pigs were given 103 pfu of Pan/99 virus intranasally and kept distant from sentinel animals until 24 h p.i. Nasal washes were performed at 48-h intervals, starting from 48 h p.i. for the inoculated animals. Cage layouts are shown in A; open, wire-top cages were placed on a shelf as shown; animals in cages 1 and 2 were infected. Viral titers in nasal wash samples are plotted in B; each curve corresponds to a single animal. “x” and “o” are used to distinguish two guinea pigs housed in the same cage; thus, 1x and 1o were housed in cage 1.
Similar articles
- Guinea pig model for evaluating the potential public health risk of swine and avian influenza viruses.
Sun Y, Bi Y, Pu J, Hu Y, Wang J, Gao H, Liu L, Xu Q, Tan Y, Liu M, Guo X, Yang H, Liu J. Sun Y, et al. PLoS One. 2010 Nov 23;5(11):e15537. doi: 10.1371/journal.pone.0015537. PLoS One. 2010. PMID: 21124850 Free PMC article. - Novel H7N9 influenza virus shows low infectious dose, high growth rate, and efficient contact transmission in the guinea pig model.
Gabbard JD, Dlugolenski D, Van Riel D, Marshall N, Galloway SE, Howerth EW, Campbell PJ, Jones C, Johnson S, Byrd-Leotis L, Steinhauer DA, Kuiken T, Tompkins SM, Tripp R, Lowen AC, Steel J. Gabbard JD, et al. J Virol. 2014 Feb;88(3):1502-12. doi: 10.1128/JVI.02959-13. Epub 2013 Nov 13. J Virol. 2014. PMID: 24227867 Free PMC article. - Transmission in the guinea pig model.
Lowen AC, Bouvier NM, Steel J. Lowen AC, et al. Curr Top Microbiol Immunol. 2014;385:157-83. doi: 10.1007/82_2014_390. Curr Top Microbiol Immunol. 2014. PMID: 25001209 Free PMC article. Review. - H5N1 hybrid viruses bearing 2009/H1N1 virus genes transmit in guinea pigs by respiratory droplet.
Zhang Y, Zhang Q, Kong H, Jiang Y, Gao Y, Deng G, Shi J, Tian G, Liu L, Liu J, Guan Y, Bu Z, Chen H. Zhang Y, et al. Science. 2013 Jun 21;340(6139):1459-63. doi: 10.1126/science.1229455. Epub 2013 May 2. Science. 2013. PMID: 23641061 - Animal models for influenza virus transmission studies: a historical perspective.
Bouvier NM. Bouvier NM. Curr Opin Virol. 2015 Aug;13:101-8. doi: 10.1016/j.coviro.2015.06.002. Epub 2015 Jun 28. Curr Opin Virol. 2015. PMID: 26126082 Free PMC article. Review.
Cited by
- Reassortment complements spontaneous mutation in influenza A virus NP and M1 genes to accelerate adaptation to a new host.
Ince WL, Gueye-Mbaye A, Bennink JR, Yewdell JW. Ince WL, et al. J Virol. 2013 Apr;87(8):4330-8. doi: 10.1128/JVI.02749-12. Epub 2013 Jan 30. J Virol. 2013. PMID: 23365443 Free PMC article. - Identification of swine influenza A virus and Stenotrophomonas maltophilia co-infection in Chinese pigs.
Hou D, Bi Y, Sun H, Yang J, Fu G, Sun Y, Liu J, Pu J. Hou D, et al. Virol J. 2012 Aug 22;9:169. doi: 10.1186/1743-422X-9-169. Virol J. 2012. PMID: 22913775 Free PMC article. - COVID-19 in Light of Seasonal Respiratory Infections.
Kiseleva I, Grigorieva E, Larionova N, Al Farroukh M, Rudenko L. Kiseleva I, et al. Biology (Basel). 2020 Aug 20;9(9):240. doi: 10.3390/biology9090240. Biology (Basel). 2020. PMID: 32825427 Free PMC article. Review. - Impact of prior seasonal H3N2 influenza vaccination or infection on protection and transmission of emerging variants of influenza A(H3N2)v virus in ferrets.
Houser KV, Pearce MB, Katz JM, Tumpey TM. Houser KV, et al. J Virol. 2013 Dec;87(24):13480-9. doi: 10.1128/JVI.02434-13. Epub 2013 Oct 2. J Virol. 2013. PMID: 24089569 Free PMC article. - Pathogenesis of 1918 pandemic and H5N1 influenza virus infections in a guinea pig model: antiviral potential of exogenous alpha interferon to reduce virus shedding.
Van Hoeven N, Belser JA, Szretter KJ, Zeng H, Staeheli P, Swayne DE, Katz JM, Tumpey TM. Van Hoeven N, et al. J Virol. 2009 Apr;83(7):2851-61. doi: 10.1128/JVI.02174-08. Epub 2009 Jan 14. J Virol. 2009. PMID: 19144714 Free PMC article.
References
- Thompson W. W., Shay D. K., Weintraub E., Brammer L., Bridges C. B., Cox N. J., Fukuda K. J. Am. Med. Assoc. 2004;292:1333–1340. - PubMed
- Dushoff J., Plotkin J. B., Viboud C., Earn D. J. D., Simonsen L. Am. J. Epidemiol. 2006;163:181–187. - PubMed
- Nichol K. L. Arch. Intern. Med. 2001;161:749–759. - PubMed
Publication types
MeSH terms
Grants and funding
- UC19 AI 062623/AI/NIAID NIH HHS/United States
- AI 58113/AI/NIAID NIH HHS/United States
- U54 AI 057158/AI/NIAID NIH HHS/United States
- U54 AI057158/AI/NIAID NIH HHS/United States
- R01 AI 18998-25/AI/NIAID NIH HHS/United States
- R01 AI018998/AI/NIAID NIH HHS/United States
- U19 AI062623/AI/NIAID NIH HHS/United States
- P01 AI058113/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical