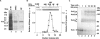PrrC-anticodon nuclease: functional organization of a prototypical bacterial restriction RNase - PubMed (original) (raw)
PrrC-anticodon nuclease: functional organization of a prototypical bacterial restriction RNase
Shani Blanga-Kanfi et al. Nucleic Acids Res. 2006.
Abstract
The tRNA(Lys) anticodon nuclease PrrC is associated in latent form with the type Ic DNA restriction endonuclease EcoprrI and activated by a phage T4-encoded inhibitor of EcoprrI. The activation also requires the hydrolysis of GTP and presence of dTTP and is inhibited by ATP. The N-proximal NTPase domain of PrrC has been implicated in relaying the activating signal to a C-proximal anticodon nuclease site by interacting with the requisite nucleotide cofactors [Amitsur et al. (2003) Mol. Microbiol., 50, 129-143]. Means described here to bypass PrrC's self-limiting translation and thermal instability allowed purifying an active mutant form of the protein, demonstrating its oligomeric structure and confirming its anticipated interactions with the nucleotide cofactors of the activation reaction. Mutagenesis and chemical rescue data shown implicate the C-proximal Arg320, Glu324 and, possibly, His356 in anticodon nuclease catalysis. This triad exists in all the known PrrC homologs but only some of them feature residues needed for tRNA(Lys) recognition by the Escherichia coli prototype. The differential conservation and consistent genetic linkage of the PrrC proteins with EcoprrI homologs portray them as a family of restriction RNases of diverse substrate specificities that are mobilized when an associated DNA restriction nuclease is compromised.
Figures
Figure 1
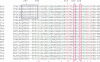
Domain organization and sequence conservation of E.coli PrrC. The degree of conservation across the sequence of E.coli PrrC was processed and color-coded by ConSeq (Pupko et al. 2002;
) based on a ClustalW alignment (45) of the query sequence with sequences of 19 homologs. These homologs are encoded by the following bacteria and indicated by the respective accession numbers and three letter abbreviations: Acidothiobacillus ferrooxidan_s (Afe), nl|TIGR_920|contig:10033:a_ferrooxidans; Actinobacillus pleuropneumoniae (Apl), gnl|OUACGT_44294|ap5.fasta.screen.Contig198; Azoarcus sp. EbN1 (Azo), NC_006513.1; Brevibacterium linens BL2 H (Bli), NZ_AAGP01000001.1|; Burkholderia pseudomallei 1655 (Bup), NZ_AAHR01000011.1; Haemophilus influenzae R2846 (Hin), ZP_00154666.1; Leptospira interrogans serovar Copenhageni str.(Lin), YP_000904.1; Magnetococcus sp. MC-1 (Mag), ZP_00289847.1: Methylobacillus flagellatus KT (Mfl), ZP_00201874.1;Neisseria meningitidis MC58_ (Nme), NP_273873.1_; Photorhabdus luminescens subsp. laumondii TTO1_ (Plu), NP_931496.1; Prosthecochloris aestuarii DSM 271 (Pae), EAN22302.1; Rhodopseudomonas palustris HaA2 (Rhp), NZ_AALQ01000003.1; Shewanella baltica OS155 (Sba), AAIO01000005.1; _Streptococcus equ_i (Seq), gnl|Sanger_1336| Contig227; Streptococcus mutans (Smu), NC_004350.1; Vibrio vulnificus YJ016 (Vvu), NP_933608.1; _Xanthomonas campestris (_Xca), NC_003902.1. Yersinia frederiksenii ATCC 33641 (Yfr), ZP_00829622.1; Motifs and functional sites indicated over the sequence include those typical of ABC transporter ATPases (41) including the Walker A and B motifs, the ABC signature motif and the switch region or linchpin His (37); the 284–294 stretch implicated in recognition of the tRNALys anticodon (–25) and the putative ACNase catalytic triad Arg320, Glu324 and His356 (this work). Conservation scale: 
 -Insufficient data—the calculation for this site was performed on <10% of the sequences.
-Insufficient data—the calculation for this site was performed on <10% of the sequences.
Figure 2
Protein levels and ACNase activities of wild-type and D222E alleles of PrrC. (A) In vivo levels of protein and ACNase cleavage products of the two PrrC forms. The expression of the two forms was induced by IPTG and the cellular levels of their protein and ACNase cleavage products were then determined as detailed in Materials and Methods. (B) In vitro ACNase activity of the indicated PrrC forms was determined as detailed in Materials and Methods. (C) Decay rates of the in vitro ACNase activity of wild-type and D222E forms of PrrC. The PrrC-D222E fraction was assayed as such or diluted 20-fold in an isogenic extract lacking PrrC. The dilution equalized the protein levels of the mutant and wild-type forms. Aliquots were pre-incubated at 37°C in the presence of 2 M TMAO and assayed for remaining ACNase activity at 10°C and 0.5 M TMAO under standard assay conditions. D222E 1:20 indicates D222E diluted 20-fold. (D) Levels of PrrC-D222E protein in the indicated E.coli strains.
Figure 3
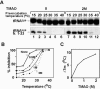
TMAO stabilizes ACNase activity. (A) PrrC-D222E aliquots were pre-incubated at the indicated temperatures for 30 min without or with 2 M TMAO. ACNase activity was assayed then for 20 min at 10°C with 1 M TMAO. (B) Inhibition of ACNase activity as a function of pre-incubation temperature and TMAO level. (C) Tm of ACNase inactivation versus TMAO level.
Figure 4
Isolation of PrrC and determination of its oligomeric structure. (A) PrrC-D222E-His6 was purified by high-speed centrifugation (S-30), immobilized cobalt affinity-chromatography (IMAC) and Superdex-200 gel filtration (GF) as detailed in Materials and Methods and Table 1. Aliquots of the indicated fractions were separated by SDS–PAGE and silver stained. (B) Superdex-200-elution profile of PrrC protein and ACNase activity. The TALON fraction of PrrC was fractionated on a Superdex-200 column. ACNase activity and PrrC protein were detected in the eluted fractions as described in Materials and Methods. The top inset shows the profile of SeeBlue stained PrrC protein, the lower inset the profile of the ACNase cleavage product of tRNALys kDa—native protein size markers. (C) GA-crosslinking profile of PrrC. The post-TALON fraction of PrrC was subjected to glutaraldehyde crosslinking. Aliquots taken at the indicated times were separated by 5–13% gradient SDS–PAGE and visualized by staining, as detailed in Materials and Methods. kDa—protein size markers. PrrC2, 3 & 4 indicate putative PrrC dimer, trimer and tetramer forms, respectively.
Figure 5
UV-crosslinking nucleotides to PrrC. (A) PrrC was incubated with the indicated concentrations of radio-labeled GTP (lanes 1–7, 0.1, 0.2, 0.4, 0.6, 0.8, 1.0 and 1.5 mM, respectively), ATP (lanes 8–14, 0.1, 0.2, 0.4, 0.6, 0.8, 1.0 and 1.5 mM, respectively) or dTTP (lanes 15–22, 0.1, 0.2, 0.4, 0.6, 0.8, 1.0, 1.5 and 2.0 μM, respectively) and UV-irradiated. The controls with and without radiation contained 0.5 mM GTP (lanes 23 and 24), 0.5 mM ATP (lanes 25 and 26) or 0.2 μM dTTP (lanes 27 and 28). The samples were separated by 10% SDS–PAGE, transferred to a nitrocellulose membrane, autoradiographed and immunoblotted (Materials and Methods). (B) Effect of heat inactivation of ACNase on PrrC's ability to UV-crosslink nucleotides and oligomeric structure. Untreated PrrC or heat inactivated aliquots (2 min at 40°C) were assayed for ACNase activity (lanes 1 and 2), glutaraldehyde protein–protein crosslinking (lanes 3–8) and UV-crosslinking the indicated nucleotides (lanes 9 and 10). (C) The indicated PrrC alleles were assayed for ability to UV-crosslink GTP (lanes 1–6), ATP (lanes 7–12) or dTTP (lanes 13–18). The asterisk in the K46N*, K168N*, K171N* and H251N* labels indicate that these mutations were introduced over the D222E background.
Figure 6
In vivo ACNase activity and protein level of PrrC mutants. The indicated PrrC mutants were compared to the wild-type allele in vivo ACNase activity by radiolabeling the resultant 5′-OH cleavage termini and for PrrC protein level by western blotting as detailed in Materials and Methods.
Figure 7
Chemical rescue of PrrC mutants. (A) The indicated PrrC alleles were assayed for ACNase activity in the presence of the indicated concentrations of guanidine-HCl or (B) ammonium acetate. (C) Relative ACNase activities versus guanidine-HCl or (D) ammonium acetate concentration.
Figure 8
PrrC residues implicated in tRNALys recognition and cleavage. The multiple sequence alignment was derived as in the legend to Figure 1. The portion shown corresponds to residues 278–335 of the E.coli query. Residues marked in red are identical, in green similar. The blue box indicates consensus sequence implicated in tRNALys recognition. The magenta box marks residues implicated in ACNase catalysis.
Similar articles
- Bacteriophage T4-encoded Stp can be replaced as activator of anticodon nuclease by a normal host cell metabolite.
Amitsur M, Benjamin S, Rosner R, Chapman-Shimshoni D, Meidler R, Blanga S, Kaufmann G. Amitsur M, et al. Mol Microbiol. 2003 Oct;50(1):129-43. doi: 10.1046/j.1365-2958.2003.03691.x. Mol Microbiol. 2003. PMID: 14507369 - Detection of anticodon nuclease residues involved in tRNALys cleavage specificity.
Meidler R, Morad I, Amitsur M, Inokuchi H, Kaufmann G. Meidler R, et al. J Mol Biol. 1999 Apr 2;287(3):499-510. doi: 10.1006/jmbi.1999.2634. J Mol Biol. 1999. PMID: 10092455 - Phage T4-coded Stp: double-edged effector of coupled DNA and tRNA-restriction systems.
Penner M, Morad I, Snyder L, Kaufmann G. Penner M, et al. J Mol Biol. 1995 Jun 23;249(5):857-68. doi: 10.1006/jmbi.1995.0343. J Mol Biol. 1995. PMID: 7791212 - Parallel dimerization of a PrrC-anticodon nuclease region implicated in tRNALys recognition.
Klaiman D, Amitsur M, Blanga-Kanfi S, Chai M, Davis DR, Kaufmann G. Klaiman D, et al. Nucleic Acids Res. 2007;35(14):4704-14. doi: 10.1093/nar/gkm494. Epub 2007 Jun 29. Nucleic Acids Res. 2007. PMID: 17604307 Free PMC article. - Anticodon nucleases.
Kaufmann G. Kaufmann G. Trends Biochem Sci. 2000 Feb;25(2):70-4. doi: 10.1016/s0968-0004(99)01525-x. Trends Biochem Sci. 2000. PMID: 10664586 Review.
Cited by
- Structure-function relations in the NTPase domain of the antiviral tRNA ribotoxin Escherichia coli PrrC.
Meineke B, Shuman S. Meineke B, et al. Virology. 2012 Jun 5;427(2):144-50. doi: 10.1016/j.virol.2012.02.008. Epub 2012 Mar 2. Virology. 2012. PMID: 22386822 Free PMC article. - Surveillance and cleavage of eukaryotic tRNAs.
Megel C, Morelle G, Lalande S, Duchêne AM, Small I, Maréchal-Drouard L. Megel C, et al. Int J Mol Sci. 2015 Jan 15;16(1):1873-93. doi: 10.3390/ijms16011873. Int J Mol Sci. 2015. PMID: 25599528 Free PMC article. Review. - RloC: a wobble nucleotide-excising and zinc-responsive bacterial tRNase.
Davidov E, Kaufmann G. Davidov E, et al. Mol Microbiol. 2008 Sep;69(6):1560-74. doi: 10.1111/j.1365-2958.2008.06387.x. Epub 2008 Aug 4. Mol Microbiol. 2008. PMID: 18681940 Free PMC article. - Alternative dimerization is required for activity and inhibition of the HEPN ribonuclease RnlA.
Garcia-Rodriguez G, Charlier D, Wilmaerts D, Michiels J, Loris R. Garcia-Rodriguez G, et al. Nucleic Acids Res. 2021 Jul 9;49(12):7164-7178. doi: 10.1093/nar/gkab513. Nucleic Acids Res. 2021. PMID: 34139012 Free PMC article. - Bacterial origins of human cell-autonomous innate immune mechanisms.
Wein T, Sorek R. Wein T, et al. Nat Rev Immunol. 2022 Oct;22(10):629-638. doi: 10.1038/s41577-022-00705-4. Epub 2022 Apr 8. Nat Rev Immunol. 2022. PMID: 35396464 Review.
References
- Yarmolinsky M.B. Programmed cell death in bacterial populations [comment] Science. 1995;267:836–837. - PubMed
- Snyder L. Phage-exclusion enzymes: a bonanza of biochemical and cell biology reagents? Mol. Microbiol. 1995;15:415–420. - PubMed
- Snyder L., Kaufmann G. T4 phage exclusion mechanisms. In: Karam J.D., Drake J.W., Kreuzer K.N., Mosig G., Hall D.W., Eiserling F.A., Black L.W., Spicer E.K., Kutter E., Carlson K., Miller E.S., editors. Molecular Biology of bacteriophage T4. Washington, D.C.: ASM Press; 1994. pp. 391–396.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases