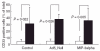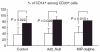Monocyte chemoattractant protein-1 (MCP-1) inhibits the intestinal-like differentiation of monocytes - PubMed (original) (raw)
Comparative Study
Monocyte chemoattractant protein-1 (MCP-1) inhibits the intestinal-like differentiation of monocytes
T Spoettl et al. Clin Exp Immunol. 2006 Jul.
Abstract
Monocytes (MO) migrating into normal, non-inflamed intestinal mucosa undergo a specific differentiation resulting in a non-reactive, tolerogenic intestinal macrophage (IMAC). Recently we demonstrated the differentiation of MO into an intestinal-like macrophage (MAC) phenotype in vitro in a three-dimensional cell culture model (multi-cellular spheroid or MCS model). In the mucosa of patients with inflammatory bowel disease (IBD) in addition to normal IMAC, a reactive MAC population as well as increased levels of monocyte chemoattractant protein 1 (MCP-1) is found. The aim of this study was to investigate the influence of MCP-1 on the differentiation of MO into IMAC. MCS were generated from adenovirally transfected HT-29 cells overexpressing MCP-1, macrophage inflammatory protein 3 alpha (MIP-3alpha) or non-transfected controls and co-cultured with freshly elutriated blood MO. After 7 days of co-culture MCS were harvested, and expression of the surface antigens CD33 and CD14 as well as the intracellular MAC marker CD68 was determined by flow-cytometry or immunohistochemistry. MCP-1 and MIP-3alpha expression by HT-29 cells in the MCS was increased by transfection at the time of MCS formation. In contrast to MIP-3alpha, MCP-1 overexpression induced a massive migration of MO into the three-dimensional aggregates. Differentiation of IMAC was disturbed in MCP-1-transfected MCS compared to experiments with non-transfected control aggregates, or the MIP-3alpha-transfected MCS, as indicated by high CD14 expression of MO/IMAC cultured inside the MCP-1-transfected MCS, as shown by immunohistochemistry and FACS analysis. Neutralization of MCP-1 was followed by an almost complete absence of monocyte migration into the MCS. MCP-1 induced migration of MO into three-dimensional spheroids generated from HT-29 cells and inhibited intestinal-like differentiation of blood MO into IMAC. It may be speculated that MCP-1 could play a role in the disturbed IMAC differentiation in IBD mucosa.
Figures
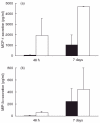
Fig. 1
Chemokine levels in supernatants of transfected cells. (a) Monocyte chemoattractant protein (MCP)-1 levels in the supernatants of non-transfected controls after 48 h of culture were 40 ± 34 pg/ml compared to 1932 ± 1631 pg/ml in HT-29 cells 48 h after Ad5_MCP-1 transfection. In supernatants of spheroids cocultured with monocyte/macrophage (MO/MAC) for 7 days the mean value of MCP-1 in the supernatant was 1016 ± 970 pg/ml in non-transfected controls compared to 4645 ± 100 pg/ml in experiments with Ad5_MCP-1-transfected cells. ▪, Control; □, Ad5_MCP-1 transfected. (b) The mean value of macrophage inflammatory protein 3 alpha (MIP-3α) in the supernatants of HT-29 cells before generation of spheroids was 8·7 ± 7·9 pg/ml in non-transfected control cells compared to 58·1 ± 23·9 pg/ml in MIP-3α-transfected cells. MIP-3α values in supernatants of multi-cellular spheroid (MCS) after 7 days of co-culture with MO/MAC were 243 ± 214 pg/ml in aggregates consisting of non-transfected HT-29 cells and 448 ± 353 pg/ml in aggregates consisting of Ad5_MIP-3α-transfected cells. ▪, Control; □, Ad5_MCP-3α transfected.
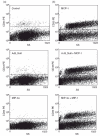
Fig. 2
Macrophages (MAC) inside the aggregates were identified by positive staining for the monocyte (MO)/MAC specific surface marker CD33. (a) CD33+ MO/MAC cultured in non-transfected, Ad5_Null- and macrophage inflammatory protein 3 alpha (MIP-3α)-transfected HT-29 multi-cellular spheroid (MCS) for 7 days. Only a low number of CD33+ cells can be detected. (b) CD33+ MO/MAC in Ad5_MCP-1-, Ad5_Null/Ad5_MCP-1- and Ad5_MIP-3α/Ad5_MCP-1-transfected HT-29 MCS after 7 days of culture. Clearly, many more CD33+ cells can be isolated from the MCS if monocyte chemoattractant protein (MCP)-1 was overexpressed.
Fig. 3
Percentage of CD33+ monocyte/macrophage (MO/MAC) in multi-cellular spheroid (MCS) after 7 days of co-culture. In control experiments with spheroids from non-transfected HT-29 cells 4·5% of total cells could be identified as MO/MAC (CD33+ cells) after 7 days (n = 11) compared to 30·9% MO/MAC of total cells in aggregates from Ad5_MCP-1-transfected cells (n = 10). In spheroids generated from HT-29 cells transfected with the empty control virus 5·1% of total cells were MO/MAC (n = 4), co-transfection of HT-29 cells with Ad5_Null and Ad5_MCP-1 resulted in 37·9% of MO/MAC inside the aggregates (n = 4). Experiments with MCS from HT-29 cells transfected with Ad5_MIP-3α revealed 4·9% CD33+ cells inside the aggregates after 7 days of co-culture (n = 7), co-transfection with Ad5_macrophage inflammatory protein 3 alpha (MIP-3α) and Ad5_MCP-1 resulted in 36·1% of CD33+ cells inside the aggregates. □, Alone; ▪, with monocyte chemoattractant protein (MCP)-1.
Fig. 4
Immunohistochemical staining of multi-cellular spheroid (MCS) co-cultured with monocytes (MO) for 7 days. Aggregates were stained for the intracellular MO/macrophage (MAC) specific marker CD68 and the differentiation and activation associated surface antigen CD14. (a–d) Non-transfected control MCS; (e–h) Ad-MCP-1-transfected MCS. (a) In non-transfected control MCS CD68+ MAC could be identified inside the aggregates after 7 days of co-culture; (b) no expression of CD14 could be detected in theses cells; (c) staining of EP4 revealed the epithelial character of the vast majority of cells; (d) isotype control staining; (e) transfection of HT-29 cells with Ad_MCP-1 resulted in a higher rate of monocyte migration into the aggregates; (f) a much higher number compared to non-transfected MCS expressed CD14; (g) EP4 staining; (h) isotype control (original magnification ×400).
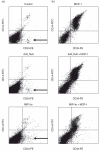
Fig. 5
Dot-plots of CD33/CD14 double-positive cells. (a) In non-transfected control spheroids 1·3% of total cells showed expression of CD33 and CD14. Transfection of HT-29 cells with Ad5_Null also resulted in 1·3% CD33+/CD14+) cells. In spheroids consisting of HT-29 cells transfected with Ad5_macrophage inflammatory protein 3 alpha (MIP-3α) 1·2% of total cells were positive for CD33 and CD14. A small population of CD33+/CD14– intestinal-like macrophages was present in all analyses (lower right quadrant, arrows). (b) In spheroids from Ad5_MCP-1-transfected cells 26·2% of total cells showed expression of CD33 and CD14 after 7 days. Co-transfection with Ad5_Null and Ad5_MCP-1 resulted in similar effects with 30·8% of total cells co-expressing CD33 and CD14. Co-transfection with Ad5_MIP-3α and Ad5_MCP-1 resulted in 34·6% of total cells expressing CD33 and CD14. No CD33+/CD14– population of cells can be detected.
Fig. 6
Percentage of CD14+ cells with respect to CD33+ cells after 7 days of co-culture. The amount of CD14+ cells among CD33+ cells was significantly higher in Ad5_MCP-1-transfected spheroids compared to aggregates from non-transfected control cells (P = 0·002, _t_-test). The same result was obtained with monocytes (MO) cultured in Ad5_Null (40·0%) and AD 5_Null/Ad5_MCP-1 (85·5%) transfected aggregates (P = 0·001, _t_-test). Also comparison between MO cultured inside Ad5_macrophage inflammatory protein 3 alpha (MIP-3α) and Ad5_MIP-3α/MCP-1 aggregates revealed a significantly higher percentage of CD14+ MO/macrophage (MAC) among CD33+ MO/MAC when HT-29 cells were transfected with Ad5_MCP1 (43·4%versus 82·6%, P = 0·013, _t_-test). □, Alone; ▪, with monocyte chemoattractant protein (MCP)-1.
Fig. 7
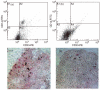
Neutralization of monocyte chemoattractant protein (MCP)-1 by the addition of anti-MCP-1 antibodies to the multi-cellular spheroid (MCS) model. A MCP-1 neutralizing antibody (R&D systems, cat. number AB-279-NA) was added to the cultures at days 1, 3 and 5 of the culture period. MCS were analysed by FACS analysis (a, b) and by immunohistochemistry (c, d). Whereas there was a high number of CD14 positive cells after transfection of HT-29 cells with Ad5_MCP-1 (a) neutralization of MCP-1 almost completely abrogated the presence of CD14+ cells in the MCS (b). For immunohistochemistry CD14 (Pharmingen, Heidelberg, Germany) was stained with APAAP (red) and CD68 (Dako; clone: KP1) was stained with BDHC (blue). The presence of CD68/CD14+ cells after Ad5_MCP-1 transfection was obvious (c), whereas no CD68/CD14 double-positive and almost no CD68 or CD14 single-positive cells could be observed after neutralization of MCP-1 (d). The experiment shown was conducted in triplicate and is representative of two further experiments.
Similar articles
- Physiological role of macrophage inflammatory protein-3 alpha induction during maturation of intestinal macrophages.
Hausmann M, Bataille F, Spoettl T, Schreiter K, Falk W, Schoelmerich J, Herfarth H, Rogler G. Hausmann M, et al. J Immunol. 2005 Aug 1;175(3):1389-98. doi: 10.4049/jimmunol.175.3.1389. J Immunol. 2005. PMID: 16034074 - A functional role for interleukin-21 in promoting the synthesis of the T-cell chemoattractant, MIP-3alpha, by gut epithelial cells.
Caruso R, Fina D, Peluso I, Stolfi C, Fantini MC, Gioia V, Caprioli F, Del Vecchio Blanco G, Paoluzi OA, Macdonald TT, Pallone F, Monteleone G. Caruso R, et al. Gastroenterology. 2007 Jan;132(1):166-75. doi: 10.1053/j.gastro.2006.09.053. Epub 2006 Oct 1. Gastroenterology. 2007. PMID: 17241869 - Monocyte chemoattractant protein 1 and macrophage cyclooxygenase 2 expression in colonic adenoma.
Tanaka S, Tatsuguchi A, Futagami S, Gudis K, Wada K, Seo T, Mitsui K, Yonezawa M, Nagata K, Fujimori S, Tsukui T, Kishida T, Sakamoto C. Tanaka S, et al. Gut. 2006 Jan;55(1):54-61. doi: 10.1136/gut.2004.059824. Epub 2005 Aug 5. Gut. 2006. PMID: 16085694 Free PMC article. - Monocyte chemoattractant protein-1 and the kidney.
Haller H, Bertram A, Nadrowitz F, Menne J. Haller H, et al. Curr Opin Nephrol Hypertens. 2016 Jan;25(1):42-9. doi: 10.1097/MNH.0000000000000186. Curr Opin Nephrol Hypertens. 2016. PMID: 26625862 Review. - The monocyte-macrophage axis in the intestine.
Bain CC, Mowat AM. Bain CC, et al. Cell Immunol. 2014 Sep-Oct;291(1-2):41-8. doi: 10.1016/j.cellimm.2014.03.012. Epub 2014 Apr 1. Cell Immunol. 2014. PMID: 24726741 Free PMC article. Review.
Cited by
- Th2 cytokine-induced alterations in intestinal smooth muscle function depend on alternatively activated macrophages.
Zhao A, Urban JF Jr, Anthony RM, Sun R, Stiltz J, van Rooijen N, Wynn TA, Gause WC, Shea-Donohue T. Zhao A, et al. Gastroenterology. 2008 Jul;135(1):217-225.e1. doi: 10.1053/j.gastro.2008.03.077. Epub 2008 Apr 4. Gastroenterology. 2008. PMID: 18471439 Free PMC article. - Genetic variations related to inflammation in suicidal ideation and behavior: A systematic review.
Tamimou R, Lumbroso S, Mouzat K, Lopez-Castroman J. Tamimou R, et al. Front Psychiatry. 2022 Oct 17;13:1003034. doi: 10.3389/fpsyt.2022.1003034. eCollection 2022. Front Psychiatry. 2022. PMID: 36325529 Free PMC article. - SerpinB2 is critical to Th2 immunity against enteric nematode infection.
Zhao A, Yang Z, Sun R, Grinchuk V, Netzel-Arnett S, Anglin IE, Driesbaugh KH, Notari L, Bohl JA, Madden KB, Urban JF Jr, Antalis TM, Shea-Donohue T. Zhao A, et al. J Immunol. 2013 Jun 1;190(11):5779-87. doi: 10.4049/jimmunol.1200293. Epub 2013 Apr 29. J Immunol. 2013. PMID: 23630350 Free PMC article. - Spherical cancer models in tumor biology.
Weiswald LB, Bellet D, Dangles-Marie V. Weiswald LB, et al. Neoplasia. 2015 Jan;17(1):1-15. doi: 10.1016/j.neo.2014.12.004. Neoplasia. 2015. PMID: 25622895 Free PMC article. Review. - CC Chemokine Receptor 4 (CCR4) as a Possible New Target for Therapy.
Bogacka J, Pawlik K, Ciapała K, Ciechanowska A, Mika J. Bogacka J, et al. Int J Mol Sci. 2022 Dec 9;23(24):15638. doi: 10.3390/ijms232415638. Int J Mol Sci. 2022. PMID: 36555280 Free PMC article. Review.
References
- Andus T, Rogler G, Daig R, Falk W, Schölmerich J, Gross V. The role of macrophages. In: Tygat GNJ, Bartelsman JFWM, van Deventer SJH, editors. Inflammatory bowel disease. The Netherlands: Kluwer Dordrecht; 1995. pp. 281–97.
- Rogler G, Andus T, Aschenbrenner E, et al. Alterations of the phenotype of colonic macrophages in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 1997;9:893–9. - PubMed
- Smith PD, Janoff EM, Mosteller-Barnum M, et al. Isolation and purification of CD14-negative mucosal macrophages from normal human small intestine. J Immunol Meth. 1997;202:1–11. - PubMed
- Smith PD, Smythies LE, Mosteller-Barnum M, et al. Intestinal macrophages lack CD14 and CD89 and consequently are down-regulated for LPS- and IgA-mediated activities. J Immunol. 2001;167:2651–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous