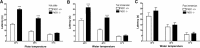Impaired nociception and inflammatory pain sensation in mice lacking the prokineticin receptor PKR1: focus on interaction between PKR1 and the capsaicin receptor TRPV1 in pain behavior - PubMed (original) (raw)
Comparative Study
Impaired nociception and inflammatory pain sensation in mice lacking the prokineticin receptor PKR1: focus on interaction between PKR1 and the capsaicin receptor TRPV1 in pain behavior
Lucia Negri et al. J Neurosci. 2006.
Abstract
Bv8, prokineticin-1 or EG-VEGF (endocrine gland-derived vascular endothelial growth factor), and prokineticin-2, are naturally occurring peptide agonists of two G-protein-coupled receptors (GPCRs), prokineticin receptor 1 (PKR1) and PKR2. PKRs are expressed in neurons in the CNS and peripheral nervous system and many dorsal root ganglion (DRG) cells expressing PKRs also express transient receptor potential vanilloid receptor-1 (TRPV1). Mice lacking the pkr1 gene were generated to explore the role of the PKR1 receptor in nociceptive signaling and in nociceptor sensitization. When compared with wild-type littermates, mice lacking the pkr1 gene showed impaired responsiveness to noxious heat, mechanical stimuli, capsaicin, and protons. In wild-type mice, activation of PKRs by the PKR agonist Bv8 caused hyperalgesia and sensitized to the actions of capsaicin. pkr1-null mice exhibited impaired responses to Bv8 but showed normal hyperalgesic responses to bradykinin and PGE2 (prostaglandin E2). Conversely, trpv1-null mice showed a reduced pronociceptive response to Bv8. Additionally, pkr1-null mice showed diminished thermal hyperalgesia after acute inflammation elicited by mustard oil and reduced pain behavior after chronic inflammation produced by complete Freund's adjuvant. The number of neurons that responded with a [Ca2+]i increase to Bv8 exposure was five times lower in pkr1-null DRG cultures than in wild-type cultures. Furthermore, Bv8-responsive neurons from pkr1-null mice showed a significant reduction in the [Ca2+]i response to capsaicin. These findings indicate a modulatory role of PKR1 in acute nociception and inflammatory pain and disclose a pharmacological interaction between PKR1 and TRPV1 in nociceptor activation and sensitization.
Figures
Figure 1.
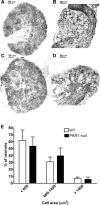
Morphology of DRG neurons in pkr1+/+ and pkr1−/− mice. A–D, Microphotographs of DRG sections from wild-type (A, B) and PKR1-null (C, D) mice, stained with _o-_toluidine blue (A, C) and immunostained with GFAP antibody (B, D). E, Size distribution of DRG neurons in WT and PKR1-null mice. Cell body area did not show significant difference between the two genotypes. For each size category, only neurons with visible nucleus were counted.
Figure 2.
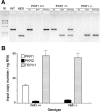
Mouse tail DNA genotyping and DRG mRNA expression of PKR genes. A, Representative example of wild-type (PKR1+/+), heterozygous mutant (PKR1+/−), and homozygous mutant (PKR1−/−) mouse tail DNA genotyped by PCR. Lane WT contains PCR amplification product of wild-type PKR1 gene; lane NEO contains PCR amplification product of the mutant gene; lanes wt contain PCR amplification products obtained with primers for wild-type PKR1 gene; lanes neo contain PCR amplification products obtained with primers for the mutant gene; lanes wt+neo indicate multiplex PCR for both WT and mutant gene; lane M contains DNA molecular weight markers. B, Expression of the genes PKR1, PKR2, and TRPV1 in DRG from wild-type (PKR1+/+) and PKR1-null mutant (PKR1−/−) mice.
Figure 3.
On-line RT-PCR of PKR1, PKR2, and TRPV1 mRNAs from laser-microdissected DRG neurons and gel electrophoresis of PCR amplification products of the three genes. A, Size distribution of neurons from wild-type and PKR1-null mice, expressing TRPV1, PKR1, and PKR2. B, Percentage of TRPV1 neurons coexpressing PKR1 or PKR2. C, Gel electrophoresis of PCR amplification products from representative samples of small, medium-sized, and large DRG neurons.
Figure 4.
In situ hybridization of DRG sections with 35S-PKR1 and 35S-PKR2 antisense riboprobe. A, C, D, F, Wild-type mice; B, E, PKR1-null mice. PKR1 is expressed by neurons smaller than those expressing PKR2. A, B, D, E, Cell profiles stained with _o-_toluidine blue; C, F, Nuclei stained with Mayer's hemalum.
Figure 5.
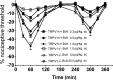
Impaired thermal nociception in PKR1-null mutant mice. A, Paw-withdrawal latency from hot plate. B, Tail-withdrawal latency from hot water were significantly longer in PKR1-null mutant mice than wild-type mice at stimulant temperature lower than 50°C. C, Hot water at mouse paw did not reveal any difference in withdrawal latency between the two genotypes. ∗∗∗p < 0.001 versus wild-type mice (2-way ANOVA with Bonferroni's post test).
Figure 6.
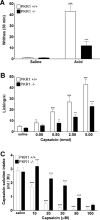
Impaired chemical nociception in pkr1-null mutant mice. A, pkr1-null mice showed a reduced number of writhes in response to intraperitoneal acetic acid compared with wild-type mice. °°°p < 0.001 versus saline; ∗∗∗p < 0.001 versus wild-type mice. B, Dose-dependent paw licking time after intraplantar capsaicin was shorter in pkr1-null mice than in wild-type mice. °p < 0.05, °°p < 0.01, °°°p < 0.001 versus vehicle; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 versus wild-type mice. C, pkr1-null mutant mice drank a larger volume of capsaicin solution than wild-type mice showing impaired trigeminal nociception. °°°p < 0.001 versus vehicle, ∗∗∗p < 0.001 versus wild-type mice (2-way ANOVA with Bonferroni's post test).
Figure 7.
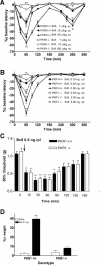
Bv8-induced hyperalgesia to heat (hot plate test), punctate stimuli (von Frey filaments), and body weight bearing (weight bearing averager) in wild-type (PKR1+/+) and pkr1-null (PKR1−/−) mice. A, Time course of thermal hyperalgesia elicited by subcutaneous doses of Bv8. B, Time course of thermal hyperalgesia elicited by intraplantar (i.pl.) doses of Bv8. C, Time course of tactile hyperalgesia measured with von Frey filaments in mice intraplantarly injected with Bv8. D, Percentage difference of body weight borne by Bv8-injected paws in wild-type mice (PKR1+/+) and PKR1-null mutant mice (PKR1−/−). ∗∗p < 0.01, ∗∗∗p < 0.001 versus saline (2-way ANOVA with Bonferroni's post test).
Figure 8.
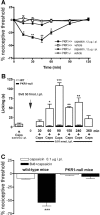
Capsaicin induced thermal hyperalgesia and Bv8 sensitized to capsaicin-induced licking and thermal hyperalgesia in WT mice and PKR1-null mice. A, Intraplantar (i.pl.) injection of 12 μg capsaicin produced an intense and long-lasting sensitization to thermal stimuli (paw immersion into 48°C water) in wild-type mice but not in PKR1-null mice. B, Intraplantar capsaicin (Caps) at dose level of 0.05 nmol induced licking in wild-type mice but not in PKR1-null mice. In contrast, a five times lower dose of capsaicin injected from 60 to 240 min after 50 fmol of Bv8 elicited a robust licking in WT mice but not in mutant mice. C, Bv8 (50 fmol) injected intraplantarly 90 min before 0.1 μg capsaicin strongly potentiated thermal hyperalgesia in wild-type mice but not in PKR1-null mice. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001 versus baseline values (2-way ANOVA with Bonferroni's post test).
Figure 9.
Impaired capsaicin (Caps.)-induced hypothermia in pkr1-null mutant mice. Hypothermic response to subcutaneous capsaicin (1 mg/kg) in wild-type mice (PKR1+/+) and in pkr1-null mutant mice (PKR1−/−). ∗p < 0.05 and ∗∗∗p < 0.001 versus pkr1-null mice (2-way ANOVA with Bonferroni's post test).
Figure 10.
Deletion of the vanilloid receptor TRPV1 gene reduced the nociceptive response to PKR activation by Bv8. The decrease in nociceptive threshold to heat produced by a subcutaneous injection of 20 μg/kg Bv8 in trpv1-null mice (TRPVR1−/−) was comparable to that obtained in wild-type mice (TRPV1+/+) by a Bv8 dose of 1 μg/kg.
Figure 11.
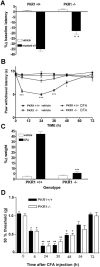
Inflammatory hyperalgesia in pkr1-null mutant mice. A, Acute inflammatory hyperalgesia induced by painting both hindpaws with mustard oil was less intense in pkr1-null mice compared with wild-type mice (hot plate test). B, C, Chronic inflammatory hyperalgesia elicited by intrapaw injection of CFA was impaired in mutant (PKR1−/−) mice (B, paw immersion in hot water; C, body weight bearing test). ∗∗p < 0.01 and ∗∗∗p < 0.001 versus wild-type mice (2-way ANOVA with Bonferroni's post test). D, In contrast, comparable tactile allodynia (von Frey filaments) was recorded in inflamed paws of both mouse genotypes after CFA injection. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001 versus baseline values (2-way ANOVA with Bonferroni's post test)
Similar articles
- Abnormal Pain Sensation in Mice Lacking the Prokineticin Receptor PKR2: Interaction of PKR2 with Transient Receptor Potential TRPV1 and TRPA1.
Maftei D, Vellani V, Artico M, Giacomoni C, Severini C, Lattanzi R. Maftei D, et al. Neuroscience. 2020 Feb 10;427:16-28. doi: 10.1016/j.neuroscience.2019.12.003. Epub 2019 Dec 26. Neuroscience. 2020. PMID: 31883821 - Sensitization of transient receptor potential vanilloid 1 by the prokineticin receptor agonist Bv8.
Vellani V, Colucci M, Lattanzi R, Giannini E, Negri L, Melchiorri P, McNaughton PA. Vellani V, et al. J Neurosci. 2006 May 10;26(19):5109-16. doi: 10.1523/JNEUROSCI.3870-05.2006. J Neurosci. 2006. PMID: 16687502 Free PMC article. - Impaired pain sensation in mice lacking prokineticin 2.
Hu WP, Zhang C, Li JD, Luo ZD, Amadesi S, Bunnett N, Zhou QY. Hu WP, et al. Mol Pain. 2006 Nov 15;2:35. doi: 10.1186/1744-8069-2-35. Mol Pain. 2006. PMID: 17107623 Free PMC article. - Bv8/Prokineticins and their Receptors A New Pronociceptive System.
Negri L, Lattanzi R, Giannini E, Canestrelli M, Nicotra A, Melchiorri P. Negri L, et al. Int Rev Neurobiol. 2009;85:145-57. doi: 10.1016/S0074-7742(09)85011-3. Int Rev Neurobiol. 2009. PMID: 19607967 Review. - Bv8-prokineticins and their receptors: modulators of pain.
Negri L, Lattanzi R. Negri L, et al. Curr Pharm Biotechnol. 2011 Oct;12(10):1720-7. doi: 10.2174/138920111798357410. Curr Pharm Biotechnol. 2011. PMID: 21466441 Review.
Cited by
- Prokineticin 2 potentiates acid-sensing ion channel activity in rat dorsal root ganglion neurons.
Qiu CY, Liu YQ, Qiu F, Wu J, Zhou QY, Hu WP. Qiu CY, et al. J Neuroinflammation. 2012 May 29;9:108. doi: 10.1186/1742-2094-9-108. J Neuroinflammation. 2012. PMID: 22642848 Free PMC article. - Preclinical Assessment of Inflammatory Pain.
Muley MM, Krustev E, McDougall JJ. Muley MM, et al. CNS Neurosci Ther. 2016 Feb;22(2):88-101. doi: 10.1111/cns.12486. Epub 2015 Dec 10. CNS Neurosci Ther. 2016. PMID: 26663896 Free PMC article. Review. - PK2/PKR1 Signaling Regulates Bladder Function and Sensation in Rats with Cyclophosphamide-Induced Cystitis.
Chen B, Zhang H, Liu L, Wang J, Ye Z. Chen B, et al. Mediators Inflamm. 2015;2015:289519. doi: 10.1155/2015/289519. Epub 2015 Dec 22. Mediators Inflamm. 2015. PMID: 26798205 Free PMC article. - Therapeutic Potential of Targeting Prokineticin Receptors in Diseases.
Vincenzi M, Kremić A, Jouve A, Lattanzi R, Miele R, Benharouga M, Alfaidy N, Migrenne-Li S, Kanthasamy AG, Porcionatto M, Ferrara N, Tetko IV, Désaubry L, Nebigil CG. Vincenzi M, et al. Pharmacol Rev. 2023 Nov;75(6):1167-1199. doi: 10.1124/pharmrev.122.000801. Epub 2023 Sep 8. Pharmacol Rev. 2023. PMID: 37684054 Free PMC article. Review. - Rapamycin ameliorates inflammatory pain via recovery of autophagy flux mediated by mammalian target of rapamycin (mTOR) signaling pathway in the rat spinal cord.
Zhang J, Chen S, Zhang R, Zheng X, Liu C, Zhang J, Zhang L, Yang Z, Wang L. Zhang J, et al. Int J Immunopathol Pharmacol. 2025 Jan-Dec;39:3946320251317284. doi: 10.1177/03946320251317284. Int J Immunopathol Pharmacol. 2025. PMID: 39895094 Free PMC article.
References
- Akopian AN, Sivilotti L, Wood JN (1996). A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature 379:257–262. - PubMed
- Angerer LM, Cox KH, Angerer RC (1987). Demonstration of tissue-specific gene expression by in situ hybridization. Methods Enzymol 152:649–661. - PubMed
- Bullock CM, Li JD, Zhou QY (2004). Structural determinants required for the bioactivities of prokineticins and identification of prokineticin receptor antagonists. Mol Pharmacol 65:582–588. - PubMed
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997). The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389:816–824. - PubMed
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D (2000). Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288:306–313. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous