Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake - PubMed (original) (raw)
. 2006 Jul;116(7):1955-62.
doi: 10.1172/JCI26532. Epub 2006 Jun 22.
Affiliations
- PMID: 16794736
- PMCID: PMC1481657
- DOI: 10.1172/JCI26532
Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake
Markus Loeffler et al. J Clin Invest. 2006 Jul.
Erratum in
- J Clin Invest. 2009 Feb;119(2):421
Abstract
Tumor-associated fibroblasts are key regulators of tumorigenesis. In contrast to tumor cells, which are genetically unstable and mutate frequently, the presence of genetically more stable fibroblasts in the tumor-stromal compartment makes them an optimal target for cancer immunotherapy. These cells are also the primary source of collagen type I, which contributes to decreased chemotherapeutic drug uptake in tumors and plays a significant role in regulating tumor sensitivity to a variety of chemotherapies. To specifically kill tumor-associated fibroblasts, we constructed an oral DNA vaccine targeting fibroblast activation protein (FAP), which is specifically overexpressed by fibroblasts in the tumor stroma. Through CD8+ T cell-mediated killing of tumor-associated fibroblasts, our vaccine successfully suppressed primary tumor cell growth and metastasis of multidrug-resistant murine colon and breast carcinoma. Furthermore, tumor tissue of FAP-vaccinated mice revealed markedly decreased collagen type I expression and up to 70% greater uptake of chemotherapeutic drugs. Most importantly, pFap-vaccinated mice treated with chemotherapy showed a 3-fold prolongation in lifespan and marked suppression of tumor growth, with 50% of the animals completely rejecting a tumor cell challenge. This strategy opens a new venue for the combination of immuno- and chemotherapies.
Figures
Figure 1. Characterization of the FAP construct and chemoresistant tumor cell lines.
(A) The cDNA encoding the entire murine FAP, verified by nucleotide sequencing, was inserted into the EcoRI site of the pFap vector. (B) Protein expression was demonstrated by Western blotting after transient transfection of CT26 and D2F2 cells. (C) CT26 colon and D2F2 breast carcinoma cells were treated with various chemotherapeutic agents at the concentrations indicated. After 48 hours of incubation, nuclear apoptosis was assessed by staining with Hoechst 33342 dye. Staurosporine was used as a positive control. PCMV, human cytomegalovirus immediate-early promoter/enhancer; BGHpA, bovine growth hormone polyadenylation signal; f1 ori, f1 origin; SV40 ori, SV40 early promoter and origin; neomycin, neomycin (G418) resistance gene; SV40pa, SV40 polyadenylation signal; pUC, pUC-derived origin; ampicillin, ampicillin resistance gene (β-lactamase).
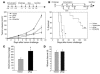
Figure 2. Effect of the FAP-based DNA vaccine on tumor growth.
(A and B) Prophylactic setting. Ten days after the last of 3 vaccinations at 1-week intervals, performed as described in Methods, BALB/c mice (n = 8) were challenged s.c. with a lethal dose of 3 × 104 CT26 cells (A) or orthotopically with a lethal dose of 3 × 105 D2F2 cells (B). The mean ± SEM of tumor growth of 8 mice is depicted. P < 0.01. (**C**) Therapeutic setting. BALB/c mice (_n_ = 8) were first injected i.v. with 105 CT26 cells and then vaccinated after 3 and 10 days once pulmonary metastases were established. After 18 days, lungs were weighed (left panel, mean + SEM), examined for metastases, and scored by a visual evaluation (right panel) assessing the percentage of lung surface covered by fused metastases as follows: 0 = 0%, 1 = <20%, 2 = 20–50%, 3 = >50%. *P < 0.01.
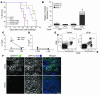
Figure 3. Antitumor effects of vaccine are mediated by CD8+ T cells.
(A) The effect on lifespan of antibody-mediated depletion during the effector phase in vaccinated mice (3 times at 1-week intervals) challenged i.v. with 105 CT26 cells. (B) CD8+ T cells were purified from spleens of vaccinated mice, stimulated with γ-irradiated tumor target cells, and then incubated for 48 hours with GFP- or GFP/pFap–transfected CT26 cells. Nuclear apoptosis was assessed by staining with Hoechst 33342 dye as follows: nuclear apoptosis stage 0, no apoptosis; stage 1, large-scale chromatin condensation; stage 2a, chromatin fragmentation; stage 2b, apoptotic bodies. (C and D) Splenocytes from pFap- and empty vector–immunized mice (n = 3) were stimulated for 5 days with pFap-transfected A31 fibroblasts and then subjected to a 51Cr-release assay. (D) Effector and target cells were coincubated with anti–MHC class I antibodies (mean + SD). (E) FACS analysis of single-cell suspensions of CT26 tumors of vaccinated mice (n = 2) stained with anti-CD3+ PerCP-Cy5.5 and anti-CD8+ FITC antibodies. One of two experiments is depicted. (F) Representative sections of CT26 tumors of pFap- and empty vector–vaccinated mice stained with anti-CD8+ FITC antibodies and DAPI nuclear stain. *Statistically significant compared with vector group, P < 0.01. **Statistically significant compared with empty vector, P < 0.05.
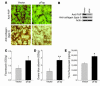
Figure 4. Expression of FAP and collagen type I and intratumoral uptake of fluorescein, albumin, and 5-fluorouracil.
(A) Immunohistochemical analysis of FAP (top) and collagen type I (bottom) expression in s.c. CT26 tumors of vaccinated mice. (B) Immunoblots of FAP and collagen type I in s.c. CT26 tumors of vaccinated mice. (C–E) Bar graphs indicate mean + SEM of OD or scintillation measurements of homogenates of s.c. CT26 tumors from vaccinated BALB/c mice (n = 4), after i.p. injection of fluorescein, i.v. injection of Evans blue albumin, or i.v. injection of 14C-5-fluorouracil. *P < 0.05, **P < 0.01.
Figure 5. Antitumor and antimetastatic effects of combined bio- and chemotherapy and side effects.
(A) Prophylactic setting. Ten days after the last of 3 vaccinations at 1-week intervals with empty vector, PBS, or pFap, BALB/c mice (n = 8, mean ± SEM) were challenged orthotopically with 3 × 105 D2F2 cells. After 5, 10, and 15 days, indicated mice were treated with doxorubicin (dox). (B) Therapeutic setting. Five days after i.v. injection of 105 D2F2 tumor cells, BALB/c mice (n = 8) were treated weekly with pFap or empty vector. One day after each immunization, mice were treated with doxorubicin i.v. as indicated (*statistically significant compared with vector group, P < 0.0001; **statistically significant compared with vector, vector/dox, and pFap groups, P < 0.0001). (C) Intratumoral doxorubicin concentration. Vaccinated BALB/c mice (n = 4) were challenged s.c. with 5 × 105 D2F2 cells, and after 16 days the doxorubicin concentration in pooled tumor lysates was determined by liquid chromatography and mass spectrometry (LC-MS) (representative of 2 experiments, mean + SD, †P < 0.001). (D) Circular wounds 3 mm in diameter were inflicted on the upper backs of vaccinated mice (n = 4), and the average time until complete wound closure was measured (mean + SD).
Similar articles
- Immunotherapy targeting fibroblast activation protein inhibits tumor growth and increases survival in a murine colon cancer model.
Wen Y, Wang CT, Ma TT, Li ZY, Zhou LN, Mu B, Leng F, Shi HS, Li YO, Wei YQ. Wen Y, et al. Cancer Sci. 2010 Nov;101(11):2325-32. doi: 10.1111/j.1349-7006.2010.01695.x. Cancer Sci. 2010. PMID: 20804499 Free PMC article. - Anti-tumor effects of DNA vaccine targeting human fibroblast activation protein α by producing specific immune responses and altering tumor microenvironment in the 4T1 murine breast cancer model.
Xia Q, Zhang FF, Geng F, Liu CL, Xu P, Lu ZZ, Yu B, Wu H, Wu JX, Zhang HH, Kong W, Yu XH. Xia Q, et al. Cancer Immunol Immunother. 2016 May;65(5):613-24. doi: 10.1007/s00262-016-1827-4. Epub 2016 Mar 28. Cancer Immunol Immunother. 2016. PMID: 27020681 Free PMC article. - Tumor-specific crosslinking of GITR as costimulation for immunotherapy.
Burckhart T, Thiel M, Nishikawa H, Wüest T, Müller D, Zippelius A, Ritter G, Old L, Shiku H, Renner C. Burckhart T, et al. J Immunother. 2010 Nov-Dec;33(9):925-34. doi: 10.1097/CJI.0b013e3181f3cc87. J Immunother. 2010. PMID: 20948444 - [FIBROBLAST ACTIVATION PROTEIN (FAP) AS A POSSIBLE TARGET OF THE ANTITUMOR STRATEGY.].
Pleshkan VV, Alekseenko IV, Tyulkina DV, Kyzmich AI, Zinovyeva MV, Sverdlov ED. Pleshkan VV, et al. Mol Gen Mikrobiol Virusol. 2016;34(3):90-97. Mol Gen Mikrobiol Virusol. 2016. PMID: 30383930 Review. Russian. - The application of the fibroblast activation protein α-targeted immunotherapy strategy.
Jiang GM, Xu W, Du J, Zhang KS, Zhang QG, Wang XW, Liu ZG, Liu SQ, Xie WY, Liu HF, Liu JS, Wu BP. Jiang GM, et al. Oncotarget. 2016 May 31;7(22):33472-82. doi: 10.18632/oncotarget.8098. Oncotarget. 2016. PMID: 26985769 Free PMC article. Review.
Cited by
- Fibroblasts as immune regulators in infection, inflammation and cancer.
Davidson S, Coles M, Thomas T, Kollias G, Ludewig B, Turley S, Brenner M, Buckley CD. Davidson S, et al. Nat Rev Immunol. 2021 Nov;21(11):704-717. doi: 10.1038/s41577-021-00540-z. Epub 2021 Apr 28. Nat Rev Immunol. 2021. PMID: 33911232 Review. - The role of cancer-associated myofibroblasts in intrahepatic cholangiocarcinoma.
Sirica AE. Sirica AE. Nat Rev Gastroenterol Hepatol. 2011 Nov 29;9(1):44-54. doi: 10.1038/nrgastro.2011.222. Nat Rev Gastroenterol Hepatol. 2011. PMID: 22143274 Review. - Short hairpin RNA targeting of fibroblast activation protein inhibits tumor growth and improves the tumor microenvironment in a mouse model.
Cai F, Li Z, Wang C, Xian S, Xu G, Peng F, Wei Y, Lu Y. Cai F, et al. BMB Rep. 2013 May;46(5):252-7. doi: 10.5483/bmbrep.2013.46.5.172. BMB Rep. 2013. PMID: 23710635 Free PMC article. - Development of a Cancer-Associated Fibroblast-Related Prognostic Model in Breast Cancer via Bulk and Single-Cell RNA Sequencing.
Hu J, Jiang Y, Wei Q, Li B, Xu S, Wei G, Li P, Chen W, Lv W, Xiao X, Lu Y, Huang X. Hu J, et al. Biomed Res Int. 2022 Dec 2;2022:2955359. doi: 10.1155/2022/2955359. eCollection 2022. Biomed Res Int. 2022. PMID: 36510567 Free PMC article. - Cancer associated fibroblasts (CAFs) in tumor microenvironment.
Xing F, Saidou J, Watabe K. Xing F, et al. Front Biosci (Landmark Ed). 2010 Jan 1;15(1):166-79. doi: 10.2741/3613. Front Biosci (Landmark Ed). 2010. PMID: 20036813 Free PMC article. Review.
References
- Marincola F.M., Wang E., Herlyn M., Seliger B., Ferrone S. Tumors as elusive targets of T-cell-based active immunotherapy. Trends Immunol. 2003;24:335–342. - PubMed
- Cohen S., Regev A., Lavi S. Small polydispersed circular DNA (spcDNA) in human cells: association with genomic instability. Oncogene. 1997;14:977–985. - PubMed
- Boehm T., Folkman J., Browder T., O’Reilly M.S. Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature. 1997;390:404–407. - PubMed
- Vitale M., et al. HLA class I antigen and transporter associated with antigen processing (TAP1 and TAP2) down-regulation in high-grade primary breast carcinoma lesions. Cancer Res. 1998;58:737–742. - PubMed
- Reed J.C. Apoptosis-targeted therapies for cancer. Cancer Cell. 2003;3:17–22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous