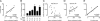Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease - PubMed (original) (raw)
Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease
Filip K Swirski et al. Proc Natl Acad Sci U S A. 2006.
Abstract
Monocytes participate importantly in the pathogenesis of atherosclerosis, but their spatial and temporal recruitment from circulation remains uncertain. This study tests the hypothesis that monocyte accumulation in atheroma correlates with the extent of disease by using a sensitive and simple quantitative assay that allows tracking of highly enriched populations of blood monocytes. A two-step isolation method yielded viable and functionally intact highly enriched peripheral blood monocyte populations (>90%). Recipient mice received syngeneic monocytes labeled in two ways: by transgenically expressing EGFP or with a radioactive tracer [(111)In]oxine. After 5 days, more labeled cells accumulated in the aorta, principally the aortic root and ascending aorta, of 10-wk-old ApoE(-/-) compared with 10-wk-old C57BL/6 mice (223 +/- 3 vs. 87 +/- 22 cells per aorta). Considerably more monocytes accumulated in 20-wk-old ApoE(-/-) mice on either chow (314 +/- 41 cells) or high-cholesterol diet (395 +/- 53 cells). Fifty-week-old ApoE(-/-) mice accumulated even more monocytes in the aortic root, ascending aorta, and thoracic aorta after both chow (503 +/- 67 cells) or high-cholesterol diet (648 +/- 81 cells). Labeled monocyte content in the aorta consistently correlated with lesion surface area. These data indicate that monocytes accumulate continuously during atheroma formation, accumulation increases in proportion to lesion size, and recruitment is augmented with hypercholesterolemia. These results provide insights into mechanisms of atherogenesis and have implications for the duration of therapies directed at leukocyte recruitment.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
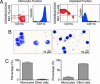
Fig. 1.
Monocyte isolation. (A) The monocyte fraction (Left) and the depleted fraction (Right) were stained with anti-CD11b, CD90, B220, DX5, NK1.1, and Ly-6G mAbs. (B) Representative cytospin preparations of the cell fractions. (C) Proportion of monocytes and other cells based on morphology and calculated by counting 10 high-power fields. Three independent experiments yielded similar results.
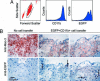
Fig. 2.
Detection of monocytes in aortic lesions. (A) Assessment of purity of isolated monocytes from EGFP-expressing mice stained with anti CD11b. (B) Monocytes were injected i.v. into 20-wk-old ApoE−/− mice on a high-cholesterol diet. Five days after injection, the aortas were excised, and immunohistochemistry was conducted with Abs against Mac-3 and EGFP. High-power fields of the lesion area at the root from mice that did not receive EGFP+ cells (Left) and from mice that received EGFP+ cells (Center and Right representing different sections) stained with anti-Mac-3 (Upper) and anti-EGFP (Lower). Data are representative of three independent experiments.
Fig. 3.
Labeling, excretion, and organ distribution of 111In species. (A) MTS assay of monocytes labeled with increasing doses of decayed [111In]oxine (squares), [111In]oxine (triangles), or [111In]chloride (inverted triangles). (B) Biodistribution of [111In]oxine-labeled monocytes compared with biodistribution of 111In alone 5 d after i.v. injection. (C) Retention of monocytes in the circulation. [111In]oxine-labeled monocytes were injected i.v., and radioactivity was measured in blood withdrawals. Biodistribution measured immediately after injection was normalized to 100%. The range of monocyte blood half-life is calculated from a 95% confidence interval, as determined from a polynomial second-order fit. (n = 2–8; mean ± SEM).
Fig. 4.
Accumulation of monocytes in the aorta. [111In]oxine-labeled monocytes were injected into WT or ApoE−/− mice (ApoE: + or −, respectively) of different age [Age (wk): 10, 20, or 50] and on either chow or high-cholesterol diets (diet C or H, respectively). Five days after injection, aortas were excised. (A) Percent injected dose per aorta. The data above the graph represent mean monocyte number ± SEM for each group. (B) Statistics (P value) for each group were calculated by using one-way ANOVA with Tukey’s multiple-comparison test.
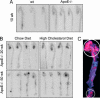
Fig. 5.
Localization of monocytes in aorta. [111In]oxine-labeled monocytes were injected into 10-wk-old WT (A Left) or ApoE−/− (A Right) mice and 20-wk-old (B Upper) and 50-wk-old (B Lower) ApoE−/− mice on chow (B Left) or high-cholesterol (B Right) diet. Five days after injection, aortas were excised, exposed to imaging plates, and subsequently read out by a PhosphorImager. Representative aortas are shown. (C) ORO staining of excised aorta from a 50-wk-old mouse on a high-cholesterol diet. Highest ORO staining typically mapped to highest radioactivity (circles).
Fig. 6.
Correlation of monocyte accumulation with serum cholesterol and lesion surface area. (A) Correlation of serum cholesterol levels with percent lesion area (n = 8). (B) Serum cholesterol levels in WT or ApoE−/− mice at different ages and on different diets, as identified in Fig. 4 (n = 4–14 per group). (C and D) Correlation between serum cholesterol and aortic accumulation in 20-wk-old (C) (n = 12) and 50-wk-old (D) (n = 14) mice, respectively. (E) Correlation of percent lesion area with aortic monocyte accumulation (n = 9). Linear regression was conducted to determine confidence of linear fit. P values are shown.
Similar articles
- Apolipoprotein A-I protection against atherosclerosis is dependent on genetic background.
Sontag TJ, Krishack PA, Lukens JR, Bhanvadia CV, Getz GS, Reardon CA. Sontag TJ, et al. Arterioscler Thromb Vasc Biol. 2014 Feb;34(2):262-9. doi: 10.1161/ATVBAHA.113.302831. Epub 2013 Dec 12. Arterioscler Thromb Vasc Biol. 2014. PMID: 24334873 - Endothelin-1 overexpression exacerbates atherosclerosis and induces aortic aneurysms in apolipoprotein E knockout mice.
Li MW, Mian MO, Barhoumi T, Rehman A, Mann K, Paradis P, Schiffrin EL. Li MW, et al. Arterioscler Thromb Vasc Biol. 2013 Oct;33(10):2306-15. doi: 10.1161/ATVBAHA.113.302028. Epub 2013 Jul 25. Arterioscler Thromb Vasc Biol. 2013. PMID: 23887640 - Foamy monocytes form early and contribute to nascent atherosclerosis in mice with hypercholesterolemia.
Xu L, Dai Perrard X, Perrard JL, Yang D, Xiao X, Teng BB, Simon SI, Ballantyne CM, Wu H. Xu L, et al. Arterioscler Thromb Vasc Biol. 2015 Aug;35(8):1787-97. doi: 10.1161/ATVBAHA.115.305609. Epub 2015 Jun 25. Arterioscler Thromb Vasc Biol. 2015. PMID: 26112011 Free PMC article. - Role of Hic-5 in the formation of microvilli-like structures and the monocyte-endothelial interaction that accelerates atherosclerosis.
Arita-Okubo S, Kim-Kaneyama JR, Lei XF, Fu WG, Ohnishi K, Takeya M, Miyauchi A, Honda H, Itabe H, Miyazaki T, Miyazaki A. Arita-Okubo S, et al. Cardiovasc Res. 2015 Mar 1;105(3):361-71. doi: 10.1093/cvr/cvv003. Epub 2015 Jan 12. Cardiovasc Res. 2015. PMID: 25587044 - Chemokine binding protein 'M3' limits atherosclerosis in apolipoprotein E-/- mice.
Ravindran D, Ridiandries A, Vanags LZ, Henriquez R, Cartland S, Tan JT, Bursill CA. Ravindran D, et al. PLoS One. 2017 Mar 10;12(3):e0173224. doi: 10.1371/journal.pone.0173224. eCollection 2017. PLoS One. 2017. PMID: 28282403 Free PMC article.
Cited by
- Macrophage Plasticity and Atherosclerosis Therapy.
Lin P, Ji HH, Li YJ, Guo SD. Lin P, et al. Front Mol Biosci. 2021 May 7;8:679797. doi: 10.3389/fmolb.2021.679797. eCollection 2021. Front Mol Biosci. 2021. PMID: 34026849 Free PMC article. Review. - The multiple roles of monocyte subsets in steady state and inflammation.
Robbins CS, Swirski FK. Robbins CS, et al. Cell Mol Life Sci. 2010 Aug;67(16):2685-93. doi: 10.1007/s00018-010-0375-x. Epub 2010 May 1. Cell Mol Life Sci. 2010. PMID: 20437077 Free PMC article. Review. - [Advances in cardiovascular medicine through molecular imaging].
Nahrendorf M, Weissleder R. Nahrendorf M, et al. Radiologe. 2007 Jan;47(1):18-24. doi: 10.1007/s00117-006-1450-z. Radiologe. 2007. PMID: 17187263 Review. German. - Melanocortin 1 Receptor Deficiency in Hematopoietic Cells Promotes the Expansion of Inflammatory Leukocytes in Atherosclerotic Mice.
Kadiri JJ, Tadayon S, Thapa K, Suominen A, Hollmén M, Rinne P. Kadiri JJ, et al. Front Immunol. 2021 Nov 19;12:774013. doi: 10.3389/fimmu.2021.774013. eCollection 2021. Front Immunol. 2021. PMID: 34868038 Free PMC article. - Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of Apoe-/- mice during disease regression.
Potteaux S, Gautier EL, Hutchison SB, van Rooijen N, Rader DJ, Thomas MJ, Sorci-Thomas MG, Randolph GJ. Potteaux S, et al. J Clin Invest. 2011 May;121(5):2025-36. doi: 10.1172/JCI43802. Epub 2011 Apr 18. J Clin Invest. 2011. PMID: 21505265 Free PMC article.
References
- Binder C. J., Chang M. K., Shaw P. X., Miller Y. I., Hartvigsen K., Dewan A., Witztum J. L. Nat. Med. 2002;8:1218–1226. - PubMed
- Libby P. Nature. 2002;420:868–874. - PubMed
- Kim C. J., Khoo J. C., Gillotte-Taylor K., Li A., Palinski W., Glass C. K., Steinberg D. Arterioscler. Thromb. Vasc. Biol. 2000;20:1976–1982. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous