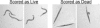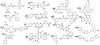Identification of novel antimicrobials using a live-animal infection model - PubMed (original) (raw)
Identification of novel antimicrobials using a live-animal infection model
Terence I Moy et al. Proc Natl Acad Sci U S A. 2006.
Abstract
The alarming increase of antibiotic-resistant bacterial pathogens points to the need for novel therapeutic approaches to combat infection. To discover novel antimicrobials, we devised a screen to identify compounds that promoted the survival of the model laboratory nematode Caenorhabditis elegans infected with the human opportunistic pathogen Enterococcus faecalis. E. faecalis colonizes the nematode intestinal tract, forming a persistent lethal infection. Infected nematodes were rescued by antibiotic treatment in a dose-dependent manner, and antibiotic treatment markedly reduced the number of bacteria colonizing the nematode intestine. To facilitate high throughput screening of compound libraries, we adapted a previously developed agar-based C. elegans-E. faecalis infection assay so that it could be carried out in liquid medium in standard 96-well microtiter plates. We used this simple infection system to screen 6,000 synthetic compounds and 1,136 natural product extracts. We identified 16 compounds and 9 extracts that promoted nematode survival. Some of the compounds and extracts inhibited E. faecalis growth in vitro, but, in contrast to traditional antibiotics, the in vivo effective dose of many of these compounds was significantly lower than the minimum inhibitory concentration needed to prevent the growth of E. faecalis in vitro. Moreover, many of the compounds and extracts had little or no affect on in vitro bacterial growth. Our findings indicate that the whole-animal C. elegans screen identifies not only traditional antibiotics, but also compounds that target bacterial virulence or stimulate host defense.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
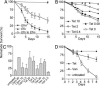
Fig. 1.
Curing of a nematode E. faecalis infection on solid medium by antibiotic treatment. (A) Kaplan–Meier survival curves of WT N2 C. elegans feeding continuously on lawns of E. faecalis (Efs) V583 (▴) or E. faecium (Efm) DO (▾). Nematodes feeding for 24 h on E. faecalis and subsequently transferred to lawns of E. faecium (□) die with an LT50 of 14.9 days. Error bars equal SEM. (B) Kaplan–Meier survival curves of E. faecalis V583-infected N2 C. elegans transferred to lawns of E. faecium on media containing tetracycline at 10 μg/ml (▴, P < 0.0001), 2 μg/ml (▿, P < 0.0014), 0.4 μg/ml (♦, P < 0.06), 0.08 μg/ml (●, P = 0.56), or no tetracycline (□). (C) Antibiotic concentrations required to promote rescue of E. faecalis VS583 infected N2 nematodes. Survival was measured 13 days postinfection after treatment with tetracycline (Tet), vancomycin (Van), or ciprofloxacin (Cipro). The MICs for the E. faecalis strain VS583 are 0.39 μg/ml Cipro, 0.27 μg/ml Tet, and 3.1 μg/ml Van. Note that VS583 is a vancomycin-sensitive derivative of V583, the well-studied vancomycin-resistant E. faecalis strain. Error bars are standard deviations. (D) Kaplan–Meier survival curve of glp-4;sek-1 nematodes infected for 12 h on E. faecalis OG1RF and transferred to BHI media containing 20 μg/ml tetracycline (▴), 40 μg/ml vancomycin (▾), or no additional antibiotic (□). Error bars equal SEM.
Fig. 2.
The liquid infection assay gauges differences in nematode killing due to E. faecalis strains with varying degrees of pathogenicity or due to antibiotic treatment. (A) Kaplan–Meier survival curves of glp-4;sek-1 nematodes that were infected with the cytolysin (Cyl) producing E. faecalis strain MMH594 (▴, ♦) or the E. faecium strain 11M12 (●). Cyl-infected nematodes were treated with 0 (▴) or 20 μg/ml (♦) tetracycline (Tet). Error bars equal SEM. (B) Kaplan–Meier survival curves of glp-4;sek-1 nematodes that were infected with E. faecalis OG1RF (▴, ♦) or the two-component quorum-sensing regulator mutant OG1RF Δ_fsrB_ (●). OG1RF-infected nematodes were treated with 0 (▴) or 20 μg/ml (♦) tetracycline. (C) The bacterial load in the nematode intestinal tract after antibiotic treatment. E. _faecalis_-infected nematodes were treated in liquid media containing 20 μg/ml ampicillin (Amp) or tetracycline, and the number of cfu per worm was determined. Error bars equal standard deviations. (D) Curing kinetics of selected hit compounds. Shown are Kaplan–Meier survival curves of infected nematodes treated with 25 μg/ml compound 4 (♦), 50 μg/ml compound 9 (●), 25 μg/ml compound 10 (□), 2.5 μg/ml tetracycline (▿), 20 μg/ml tetracycline (▴), or mock treatment (■). In pairwise comparisons to mock treatment using log-rank tests, the difference for all of the treatments was significant, with P < 0.0001.
Fig. 3.
Scoring live/dead worms in the liquid killing assay. Living nematodes in the liquid infection assay maintain a sinusoidal shape, whereas dead nematodes in the liquid infection assay appear as straight, rigid rods as the corpse becomes filled with bacteria.
Fig. 4.
Structures of the compounds that promote the survival of nematodes infected with E. faecalis. The compound number in bold corresponds to the numbering in Table 1.
Similar articles
- The worm turns for antimicrobial discovery.
Bhavsar AP, Brown ED. Bhavsar AP, et al. Nat Biotechnol. 2006 Sep;24(9):1098-100. doi: 10.1038/nbt0906-1098. Nat Biotechnol. 2006. PMID: 16964218 No abstract available. - High-throughput screen for novel antimicrobials using a whole animal infection model.
Moy TI, Conery AL, Larkins-Ford J, Wu G, Mazitschek R, Casadei G, Lewis K, Carpenter AE, Ausubel FM. Moy TI, et al. ACS Chem Biol. 2009 Jul 17;4(7):527-33. doi: 10.1021/cb900084v. ACS Chem Biol. 2009. PMID: 19572548 Free PMC article. - Novel Immune Modulators Enhance Caenorhabditis elegans Resistance to Multiple Pathogens.
Hummell NA, Revtovich AV, Kirienko NV. Hummell NA, et al. mSphere. 2021 Jan 6;6(1):e00950-20. doi: 10.1128/mSphere.00950-20. mSphere. 2021. PMID: 33408224 Free PMC article. - The worm has turned--microbial virulence modeled in Caenorhabditis elegans.
Sifri CD, Begun J, Ausubel FM. Sifri CD, et al. Trends Microbiol. 2005 Mar;13(3):119-27. doi: 10.1016/j.tim.2005.01.003. Trends Microbiol. 2005. PMID: 15737730 Review. - Molecular Mechanism of Quorum-Sensing in Enterococcus faecalis: Its Role in Virulence and Therapeutic Approaches.
Ali L, Goraya MU, Arafat Y, Ajmal M, Chen JL, Yu D. Ali L, et al. Int J Mol Sci. 2017 May 3;18(5):960. doi: 10.3390/ijms18050960. Int J Mol Sci. 2017. PMID: 28467378 Free PMC article. Review.
Cited by
- Targeting Multidrug-resistant Staphylococci with an anti-rpoA Peptide Nucleic Acid Conjugated to the HIV-1 TAT Cell Penetrating Peptide.
Abushahba MF, Mohammad H, Seleem MN. Abushahba MF, et al. Mol Ther Nucleic Acids. 2016 Jul 19;5(7):e339. doi: 10.1038/mtna.2016.53. Mol Ther Nucleic Acids. 2016. PMID: 27434684 Free PMC article. - Platforms for antibiotic discovery.
Lewis K. Lewis K. Nat Rev Drug Discov. 2013 May;12(5):371-87. doi: 10.1038/nrd3975. Nat Rev Drug Discov. 2013. PMID: 23629505 Review. - The Antioxidant Capacity In Vitro and In Vivo of Polysaccharides From Bergenia emeiensis.
Zeng C, Feng S. Zeng C, et al. Int J Mol Sci. 2020 Oct 9;21(20):7456. doi: 10.3390/ijms21207456. Int J Mol Sci. 2020. PMID: 33050354 Free PMC article. - The Drosophila melanogaster host model.
Igboin CO, Griffen AL, Leys EJ. Igboin CO, et al. J Oral Microbiol. 2012;4. doi: 10.3402/jom.v4i0.10368. Epub 2012 Feb 21. J Oral Microbiol. 2012. PMID: 22368770 Free PMC article. - Intraspecies variation in the emergence of hyperinfectious bacterial strains in nature.
Heithoff DM, Shimp WR, House JK, Xie Y, Weimer BC, Sinsheimer RL, Mahan MJ. Heithoff DM, et al. PLoS Pathog. 2012;8(4):e1002647. doi: 10.1371/journal.ppat.1002647. Epub 2012 Apr 12. PLoS Pathog. 2012. PMID: 22511871 Free PMC article.
References
- Zinner S. H. Expert Rev. Anti Infect. Ther. 2005;3:907–913. - PubMed
- Molbak K. Clin. Infect. Dis. 2005;41:1613–1620. - PubMed
- Wisplinghoff H., Bischoff T., Tallent S. M., Seifert H., Wenzel R. P., Edmond M. B. Clin. Infect. Dis. 2004;39:309–317. - PubMed
- Lane H. C., Montagne J. L., Fauci A. S. Nat. Med. 2001;7:1271–1273. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical