Large store-operated calcium selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor, Stim1 - PubMed (original) (raw)
Large store-operated calcium selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor, Stim1
Jason C Mercer et al. J Biol Chem. 2006.
Abstract
The molecular nature of store-operated Ca(2+)-selective channels has remained an enigma, due largely to the continued inability to convincingly demonstrate Ca(2+)-selective store-operated currents resulting from exogenous expression of known genes. Recent findings have implicated two proteins, Stim1 and Orai1, as having essential roles in store-operated Ca(2+) entry across the plasma membrane. However, transient overexpression of these proteins on their own results in little or no increase in store-operated entry. Here we demonstrate dramatic synergism between these two mediators; co-transfection of HEK293 cells with Stim1 and Orai1 results in an approximate 20-fold increase in store-operated Ca(2+) entry and Ca(2+)-selective current. This demonstrates that these two proteins are limiting for both the signaling and permeation mechanisms for Ca(2+)-selective store-operated Ca(2+) entry. There are three mammalian homologs of Orai1, and in expression experiments they all produced or augmented store-operated Ca(2+) entry with efficacies in the order Orai1 > Orai2 > Orai3. Stim1 apparently initiates the signaling process by acting as a Ca(2+) sensor in the endoplasmic reticulum. This results in rearrangement of Stim1 within the cell and migration toward the plasma membrane to regulate in some manner Orai1 located in the plasma membrane. However, we demonstrate that Stim1 does not incorporate in the surface membrane, and thus likely regulates or interacts with Orai1 at sites of close apposition between the plasma membrane and an intracellular Stim1-containing organelle.
Figures
Figure 1
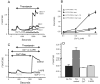
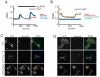
Characterization of thapsigargin-induced Ca2+ entry in HEK293 cells transfected with Orai1 and Stim1. (A) HEK293 cells transfected with combinations of EYFP (control), Stim1-EYFP and Orai1 were loaded with the fluorescent Ca2+ indicator fura-5F. Before treatment with thapsigargin (2 μM), the HBSS bathing the cells was switched from one containing 1.8 mM Ca2+ to one that was nominally Ca2+-free. After the thapsigargin-induced [Ca2+]i transient had returned to basal levels, the extracellular Ca2+ concentration was sequentially elevated (in mM; 0.1, 0.3, 1.0, 1.8), each for a 5 minute period. The experiment shown is representative of 3 similar experiments, and each trace is an average of data from 20-30 cells. (B) Summarized means ± SEM of peak [Ca2+]i levels at each Ca2+ concentration from three experiments, 20-30 cells per coverslip, for the protocol represented in (A). Filled circles show responses to Ca2+ concentrations in thapsigargin-treated cells transfected with EYFP-Stim1 and Orai1. The control data are for EYFP-Stim1 transfected cells (no Orai1, open triangles) Also shown are data for leak (no thapsigargin) in EYFP-Stim1 transfected (filled triangle) and EYFP-Stim1 + Orai1 transfected (open circles) cells. (C) Ca2+ entry in EYFP-Stim1-expressing cells is blocked by 1 μM Gd3+ or 30 μM 2APB. The data shown in C contains averaged traces from 3 experiments, with each experiment an average of data from 20 - 30 cells. (D) Summarized means ± SEM of peak [Ca2+]i levels from three experiments, 20-30 cells per coverslip, for the protocol represented in (C).
Figure 2
IP3- and BAPTA-mediated whole-cell currents in isolated HEK293 cells co-expressing Stim1 and Orai1. (A) Average time course of SOC current development after activation with 100 μM inositol 1,4,5-trisphosphate (IP3) and 10 mM BAPTA in the pipette solution in HEK293 cells expressing YFP alone (Control: black dashed trace), Orai1 (Orai1 alone: grey dashed trace), Orai1 and Stim1 (Orai1 + Stim1: black trace), or Orai1 and Stim1 under the presence of 1 μM Gd3+ (Orai1 + Stim1 + 1 μM Gd3+: grey trace). External solution contained 10 mM Ca2+ in these experiments to keep experimental conditions consistent between YFP alone and overexpressing cells. (B) Same as (A), except the internal stores were passively depleted with only 10 mM BAPTA in the intracellular pipette solution. (C) Current-voltage relationship recorded from HEK293 cells expressing only YFP (Control: black trace), Orai1 and Stim1 depleted of their internal Ca2+ stores by the addition of 100 μM IP3 and 10 mM BAPTA (Orai1 + Stim1-IP3: black trace), or 10 mM BAPTA alone (Orai1 + Stim1-BAPTA: grey trace). (D) Representative time course of the transient divalent-free potentiation of Orai1 + Stim1 currents, followed by the inhibitory effect of nominally Ca2+ free conditions (NCF) on the current recorded at −100 mV (n=7). (E) Typical time course showing both the potentiation at low (5 μM) and inhibition at high (30 μM) concentration of 2APB (n=3). Error bars indicate mean ± SEM (n=7 for Orai1 + Stim1 in A, n=5 for Orai1 + Stim1 in B, all others n=5).
Figure 3
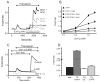
Overexpression of Orai2 and Stim1 augments thapsigargin-induced Ca2+ entry. (A) HEK293 cells transfected with combinations of EYFP (control), EYFP-Stim and Orai2 were loaded with the fluorescent Ca2+ indicator fura-5F. Before treatment with thapsigargin (2 μM), the HBSS bathing the cells was switched from one containing 1.8 mM Ca2+ to one that was nominally Ca2+-free. After the thapsigargin-induced [Ca2+]i transient had returned to basal levels, the extracellular Ca2+ concentration was sequentially elevated (in mM; 0.1, 0.3, 1.0, 1.8), each for a 5 minute period. The experiment shown is representative of 3 similar experiments, and each trace is an average of data from 20-30 cells. For comparison, data for EYFP-Stim1 + Orai1 (from Figure 1) are included for comparison. (B) Summarized means ± SEM of peak [Ca2+]i levels at each Ca2+ concentration from three coverslips, 20-30 cells per coverslip, for the protocol represented in (A). (C) Ca2+ entry in EYFP-Stim1-expressing cells is blocked by 1 μM Gd3+ or 30 μM 2APB. For this experiment, to increase the Ca2+ entry response, 2.0 μg/well of plasmid encoding Orai2 was used, where as for the experiments in (A) and (B), the same amount of plasmid was used as for Orai1, 0.5 μg per well. The data shown contains averaged traces from 3 experiments, with each experiment an average of data from 20 - 30 cells. (D) Summarized means ± SEM of peak [Ca2+]i levels from three separate experiments for the protocol represented in (C).
Figure 4
Whole cell currents in cells expressing EYFP-Stim1 and Orai2. (A) Average time course of current development at −100 mV for HEK293 cells overexpressing both Orai2 and Stim1 (n=10, ± SEM). Currents were activated by store depletion using IP3 and BAPTA in the pipette, and were recorded under the presence of 2 mM Ca2+ in the bathing solution. (B) Representative time course (n=9) of current development under the presence of 2 mM Ca2+ followed by the addition of divalent-free solution (0.1 mM EGTA) (DVF) and nominally Ca2+ free external solution (NCF). (C) Current-Voltage relationship taken from the same recording seen in (B) showing the inwardly rectifying Orai2 + Stim1 currents that are transiently potentiated by switching to DVF conditions.
Figure 5
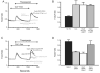
Orai3 rescues thapsigargin-activated Ca2+ entry after RNAi knockdown of Orai1. (A) HEK293 cells transfected with combinations of Stim1-EYFP (control) and Orai3 plus Stim1-EYFP. In the latter condition, the concentration of Orai3 cDNA used was either 0.5 μg or 2 μg per well of a 6-well plate. Transfected cells were loaded with the fluorescent calcium indicator fura-5F. Before treatment with thapsigargin (2 μM), the HBSS bathing the cells was switched from one containing 1 mM Ca2+ to one that was nominally Ca2+-free. After the thapsigargin-induced [Ca2+]i transient had returned to basal levels, the extracellular calcium concentration was elevated to 1 mM. The traces shown contain averaged traces from 3 similar experiments, and each experiment is an average of data from 20-30 cells. (B) Means ± SEM of the peak increase upon Ca2+ addition for the experiments in (A). (C) As described in Methods, HEK293 cells were transfected with Orai1-siRNA and siGLO. Two days post transfection, a subset of these cells were subsequently transfected with cDNA for EYFP, Stim1-EYFP or Orai3 plus EYFP. The concentration of Orai3 cDNA used in these experiments was 2 μg per well of a 6-well plate. 48 hours after cDNA transfection, cells attached to coverslips were then loaded with fura-5F and treated with thapsigargin in the absence and then the presence of 1.8 mM extracellular Ca2+. The traces shown contain averaged traces from 3 similar experiments, and each experiment is an average of data from 20-30 cells. (D) Means ± SEM of the peak increase upon Ca2+ addition for all three experiments in (C).
Figure 6
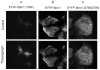
Cellular movements of the Ca2+ sensor, Stim1. A: A field of several HEK293 cells expressing EYFP-Stim1 was imaged by TIRFM just prior to (upper panel) and 6.5 min after (lower panel) depletion of intracellular Ca2+ stores with thapsigargin (2 μM) in nominally Ca2+ free extracellular medium (n = greater than 10 coverslips). B,C: A field of several HEK293 cells expressing EYFP-Stim1 (B) or EYFP-Stim1-D76N, D78N (C) was imaged by fluorescence confocal microscopy just prior to (upper panel) and 10 min after (lower panel) depletion of intracellular Ca2+ stores with thapsigargin (2 μM) in HBSS containing 1.8 mM Ca2+. For B and C, data are representative of 3 independent experiments.
Figure 7
EYFP-Stim1 or EYFP-Stim1-D76A are not detected at the cell surface by confocal microscopy. (A) HEK293 cells were transfected with siRNA directed against Stim1. Two days post transfection, a subset of these cells was transfected with EYFP or EYFP-Stim1. 24 hours later, cells were loaded with Fura-5F and their ability to activate SOC influx was assessed by single-cell Ca2+ imaging. Shown are average traces from at least 20-30 cells per trace. (B) HEK293 cells were transfected with EYFP, EYFP-Stim1, EYFP-Stim1-D76A, or EYFP-STIM1-D76N,D78N. Cells were loaded with Fura-5F/AM and their intracellular Ca2+ content was assessed by single-cell Ca2+ imaging. Initially, cells were kept in buffer containing 1.8 mM CaCl2, cells were then switched to buffer nominally free of Ca2+, and finally the buffer was exchanged again with buffer containing 1.8 mM CaCl2. Shown are average traces from at least 20-30 cells per trace. (C) and (D) HEK293 cells were transfected with N-terminally tagged EYFP-Stim1 (C) or EYFP-Stim1-D76A (D). Before fixation and permeabilization with Triton X-100 (bottom panels only), a subset of cells was treated with thapsigargin for 15 minutes in Ca2+ free PBS to deplete intracellular Ca2+ stores. Cells were then stained with anti EYFP Alexa 647-conjugated antibody. Cells were then analyzed by laser scanning confocal microscopy to detect EYFP and/or Alexa 647.
Figure 8
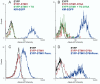
Extracellular EYFP-Stim1 is not detected by flow cytometry. (A) Jurkat cells were transfected with EYFP, N-terminal tagged EYFP-Stim1, or KIR-EGFP, an NK cell receptor with GFP fused to the extracellular domain. The next day, EYFP-Stim1 cells were either left untreated in Ca2+ containing buffer or treated with thapsigargin for 15 minutes in the absence of Ca2+. All cells were then gently fixed on ice with 0.25% para-formaldehyde to prevent additional biological changes. Cells were then stained with anti-EYFP Alexa 647 and analyzed by flow cytometry. Histograms shown are gated on the EGFP/EYFP+ portion of the viable cells. (B) Jurkat cells were transfected with EYFP, EYFP-Stim1-D76A or KIR-EGFP and treated as in (A). (C) and (D) Jurkat cells expressing either EYFP-Stim1 (C), or EYFP-Stim1-D76A (D) were either left intact or permeabilized with Triton X-100 prior to antibody staining and analysis by flow cytometry.
Figure 9
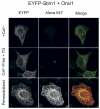
When co-expressed with Orai1, EYFP-Stim1 is not detected at the cell surface by confocal microscopy. HEK293 cells transfected with EYFP-Stim1 and Orai1 cDNA were incubated in PBS with 1.8 mM Ca2+ (top and bottom panels), or in Ca2+-free PBS containing 2 μM thapsigargin for 15 minutes prior to fixation and permeabilization with triton X-100 (bottom panels only). Cells were stained with anti-EYFP Alexa 647 conjugated antibody and analyzed by laser scanning confocal microscopy to detect EYFP and/or Alexa 647.
Similar articles
- Orai1 and STIM reconstitute store-operated calcium channel function.
Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W, Gill DL. Soboloff J, et al. J Biol Chem. 2006 Jul 28;281(30):20661-20665. doi: 10.1074/jbc.C600126200. Epub 2006 Jun 9. J Biol Chem. 2006. PMID: 16766533 - Orai1-Orai2 complex is involved in store-operated calcium entry in chondrocyte cell lines.
Inayama M, Suzuki Y, Yamada S, Kurita T, Yamamura H, Ohya S, Giles WR, Imaizumi Y. Inayama M, et al. Cell Calcium. 2015 May;57(5-6):337-47. doi: 10.1016/j.ceca.2015.02.005. Epub 2015 Feb 19. Cell Calcium. 2015. PMID: 25769459 - Calcium inhibition and calcium potentiation of Orai1, Orai2, and Orai3 calcium release-activated calcium channels.
DeHaven WI, Smyth JT, Boyles RR, Putney JW Jr. DeHaven WI, et al. J Biol Chem. 2007 Jun 15;282(24):17548-56. doi: 10.1074/jbc.M611374200. Epub 2007 Apr 23. J Biol Chem. 2007. PMID: 17452328 - Store-Independent Orai Channels Regulated by STIM.
Zhang X, Gueguinou M, Trebak M. Zhang X, et al. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 11. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 11. PMID: 30299650 Free Books & Documents. Review. - The STIM/Orai coupling machinery.
Frischauf I, Schindl R, Derler I, Bergsmann J, Fahrner M, Romanin C. Frischauf I, et al. Channels (Austin). 2008 Jul-Aug;2(4):261-8. doi: 10.4161/chan.2.4.6705. Epub 2008 Jul 21. Channels (Austin). 2008. PMID: 18769136 Review.
Cited by
- The STIM1-ORAI1 microdomain.
Hogan PG. Hogan PG. Cell Calcium. 2015 Oct;58(4):357-67. doi: 10.1016/j.ceca.2015.07.001. Epub 2015 Jul 17. Cell Calcium. 2015. PMID: 26215475 Free PMC article. Review. - Multifaceted roles of STIM proteins.
Hooper R, Samakai E, Kedra J, Soboloff J. Hooper R, et al. Pflugers Arch. 2013 Oct;465(10):1383-96. doi: 10.1007/s00424-013-1270-8. Epub 2013 Apr 9. Pflugers Arch. 2013. PMID: 23568369 Free PMC article. Review. - Development of Store-Operated Calcium Entry-Targeted Compounds in Cancer.
Liang X, Zhang N, Pan H, Xie J, Han W. Liang X, et al. Front Pharmacol. 2021 May 28;12:688244. doi: 10.3389/fphar.2021.688244. eCollection 2021. Front Pharmacol. 2021. PMID: 34122115 Free PMC article. Review. - Molecular biophysics of Orai store-operated Ca2+ channels.
Amcheslavsky A, Wood ML, Yeromin AV, Parker I, Freites JA, Tobias DJ, Cahalan MD. Amcheslavsky A, et al. Biophys J. 2015 Jan 20;108(2):237-46. doi: 10.1016/j.bpj.2014.11.3473. Biophys J. 2015. PMID: 25606672 Free PMC article. Review. - STIM2 protein mediates distinct store-dependent and store-independent modes of CRAC channel activation.
Parvez S, Beck A, Peinelt C, Soboloff J, Lis A, Monteilh-Zoller M, Gill DL, Fleig A, Penner R. Parvez S, et al. FASEB J. 2008 Mar;22(3):752-61. doi: 10.1096/fj.07-9449com. Epub 2007 Sep 28. FASEB J. 2008. PMID: 17905723 Free PMC article.
References
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. Nature. 2006 advanced online publication. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous