Hepatitis C virus entry depends on clathrin-mediated endocytosis - PubMed (original) (raw)
Hepatitis C virus entry depends on clathrin-mediated endocytosis
Emmanuelle Blanchard et al. J Virol. 2006 Jul.
Abstract
Due to difficulties in cell culture propagation, the mechanisms of hepatitis C virus (HCV) entry are poorly understood. Here, postbinding cellular mechanisms of HCV entry were studied using both retroviral particles pseudotyped with HCV envelope glycoproteins (HCVpp) and the HCV clone JFH-1 propagated in cell culture (HCVcc). HCVpp entry was measured by quantitative real-time PCR after 3 h of contact with target cells, and HCVcc infection was quantified by immunoblot analysis and immunofluorescence detection of HCV proteins expressed in infected cells. The functional role of clathrin-mediated endocytosis in HCV entry was assessed by small interfering RNA-mediated clathrin heavy chain depletion and with chlorpromazine, an inhibitor of clathrin-coated pit formation at the plasma membrane. In both conditions, HCVpp entry and HCVcc infection were inhibited. HCVcc infection was also inhibited by pretreating target cells with bafilomycin A1 or chloroquine, two drugs known to interfere with endosome acidification. These data indicate that HCV enters target cells by clathrin-mediated endocytosis, followed by a fusion step from within an acidic endosomal compartment.
Figures
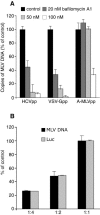
FIG. 1.
PCR-based HCVpp entry assay. (A) PLC/PRF/5 cells were pretreated for 30 min with 20, 50, or 100 nM bafilomycin A1 or left untreated. The cells were then infected with HCVpp, VSV-Gpp, or A-MLVpp in the presence of the drug. After 2 h of spinoculation and an additional hour of incubation, total DNA was purified and retroviral DNA was quantified by real-time PCR. Mean values for the controls with no drug were 1.2 equivalent genomes per cell (EG/cell) for HCVpp, 1.5 EG/cell for VSV-Gpp, and 1.3 EG/cell for A-MLVpp infections. The background value obtained from cells infected with pseudotyped particles produced in the absence of envelope proteins corresponded to 0.03 EG/cell. Results were expressed as the percentage of entry in control cells treated with no drug. (B) PLC/PRF/5 cells were infected by spinoculation with different dilutions of the same HCVpp stock. Cells were either immediately lysed to extract DNA or cultured for 3 days to allow for luciferase expression. Retroviral DNA was quantified by real-time PCR (MLV DNA), and infection was quantified by luciferase assay (Luc). Mean values for the undiluted HCVpp infection were 1.3 EG/cell and 6.5 × 106 relative light units (RLU), and the background values obtained from cells infected with pseudotyped particles produced in the absence of envelope proteins corresponded to 0.018 EG/cell and 3.0 × 102 RLU. Results were expressed as the percentage of entry in cells infected with undiluted HCVpp stock (1:1).
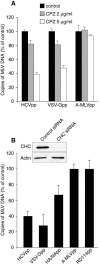
FIG. 2.
Clathrin-mediated entry of HCVpp. (A) PLC/PRF/5 cells were pretreated for 30 min with 2 or 5 μg/ml chlorpromazine (CPZ) or were left untreated. The cells were then infected with HCVpp, VSV-Gpp, or A-MLVpp by spinoculation in the presence of the drug. (B) PLC/PRF/5 cells were transfected twice with CHC siRNA or control siRNA, as explained in Materials and Methods. Two days after the second siRNA transfection, cells were spinoculated with HCVpp, VSV-Gpp, HA/NApp, RD114pp, or A-MLVpp. The cellular content of retroviral DNA was quantified by real-time PCR as a measure of entry and is expressed as a percentage of entry in untreated control cells (A) or in cells treated with control siRNA (B). The inset in panel B shows an immunoblot of the relative CHC and actin contents in siRNA-treated cells.
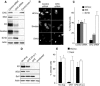
FIG. 3.
Clathrin-mediated entry of HCVcc. (A to C) Huh-7 cells were treated with CHC or control siRNA and then infected with HCVcc, a recombinant Sindbis virus expressing HCV E1, or SV40. (A) Cells were lysed at 5 hpi (Sindbis) or 30 hpi (HCVcc and SV40), and the cell lysates were analyzed by immunoblotting with antibodies to E2, NS3 (HCVcc), E1 (Sindbis), T-antigen (SV40), CHC, and actin. (B) Cells were fixed and processed for immunofluorescent detection of E2 (HCVcc), E1 (Sindbis), or T antigen (SV40). (B) For each virus, the fields presented contain similar numbers of CHC or control siRNA-treated cells. (C) Percentage of infected cells in CHC and control siRNA-treated cells. (D and E) Huh-7 cells were pretreated for 30 min and then infected for 2 h with HCVcc in the presence or the absence of chlorpromazine (CPZ; 5 μg/ml). Cells infected in the absence of drug were cultured with no drug, or chlorpromazine was added 2 hpi (CPZ 2hpi). Infection was analyzed (D) by immunoblotting with antibodies to E2, NS3, GFP (SeV), and actin or (E) by immunofluorescent detection of infected cells using an anti-E2 MAb (HCVcc) or by GFP fluorescence (SeV). Results are presented as percentages of infected cells.
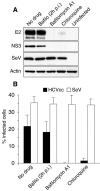
FIG. 4.
pH dependency of HCVcc infection. Huh-7 cells were pretreated for 30 min and then infected with HCVcc or SeV in the presence of 50 nM bafilomycin A1 or 20 μM chloroquine or in the absence of any inhibitor of endosomal acidification. Cells infected in the absence of drug were cultured with no drug, or bafilomycin A1 was added 2 hpi (Bafilo 2hpi). HCVcc infection was analyzed (A) by immunoblotting with antibodies to E2, NS3, GFP (SeV) and actin or (B) by immunofluorescent detection of infected cells using an anti-E2 MAb (HCVcc) or by GFP fluorescence (SeV). Results are presented as percentages of infected cells.
Similar articles
- Clathrin mediates infectious hepatitis C virus particle egress.
Benedicto I, Gondar V, Molina-Jiménez F, García-Buey L, López-Cabrera M, Gastaminza P, Majano PL. Benedicto I, et al. J Virol. 2015 Apr;89(8):4180-90. doi: 10.1128/JVI.03620-14. Epub 2015 Jan 28. J Virol. 2015. PMID: 25631092 Free PMC article. - Hepatitis C virus entry requires a critical postinternalization step and delivery to early endosomes via clathrin-coated vesicles.
Meertens L, Bertaux C, Dragic T. Meertens L, et al. J Virol. 2006 Dec;80(23):11571-8. doi: 10.1128/JVI.01717-06. Epub 2006 Sep 27. J Virol. 2006. PMID: 17005647 Free PMC article. - Arbidol inhibits viral entry by interfering with clathrin-dependent trafficking.
Blaising J, Lévy PL, Polyak SJ, Stanifer M, Boulant S, Pécheur EI. Blaising J, et al. Antiviral Res. 2013 Oct;100(1):215-9. doi: 10.1016/j.antiviral.2013.08.008. Epub 2013 Aug 25. Antiviral Res. 2013. PMID: 23981392 - Similarities and Differences Between HCV Pseudoparticle (HCVpp) and Cell Culture HCV (HCVcc) in the Study of HCV.
Riva L, Dubuisson J. Riva L, et al. Methods Mol Biol. 2019;1911:33-45. doi: 10.1007/978-1-4939-8976-8_2. Methods Mol Biol. 2019. PMID: 30593616 Review. - The hepatitis C virus and its hepatic environment: a toxic but finely tuned partnership.
Perrault M, Pécheur EI. Perrault M, et al. Biochem J. 2009 Oct 12;423(3):303-14. doi: 10.1042/BJ20091000. Biochem J. 2009. PMID: 19807698 Review.
Cited by
- Clathrin mediates infectious hepatitis C virus particle egress.
Benedicto I, Gondar V, Molina-Jiménez F, García-Buey L, López-Cabrera M, Gastaminza P, Majano PL. Benedicto I, et al. J Virol. 2015 Apr;89(8):4180-90. doi: 10.1128/JVI.03620-14. Epub 2015 Jan 28. J Virol. 2015. PMID: 25631092 Free PMC article. - The missing pieces of the HCV entry puzzle.
Ogden SC, Tang H. Ogden SC, et al. Future Virol. 2015;10(4):415-428. doi: 10.2217/FVL.15.12. Future Virol. 2015. PMID: 25960762 Free PMC article. - PmAP2-β depletion enhanced activation of the Toll signaling pathway during yellow head virus infection in the black tiger shrimp Penaeus monodon.
Jatuyosporn T, Laohawutthichai P, Supungul P, Sotelo-Mundo RR, Ochoa-Leyva A, Tassanakajon A, Krusong K. Jatuyosporn T, et al. Sci Rep. 2021 May 18;11(1):10534. doi: 10.1038/s41598-021-89922-w. Sci Rep. 2021. PMID: 34006863 Free PMC article. - Transcriptional Regulatory Networks in Hepatitis C Virus-induced Hepatocellular Carcinoma.
Zahra M, Azzazy H, Moustafa A. Zahra M, et al. Sci Rep. 2018 Sep 24;8(1):14234. doi: 10.1038/s41598-018-32464-5. Sci Rep. 2018. PMID: 30250040 Free PMC article. - New treatments for chronic hepatitis C.
Jang JY, Chung RT. Jang JY, et al. Korean J Hepatol. 2010 Sep;16(3):263-77. doi: 10.3350/kjhep.2010.16.3.263. Korean J Hepatol. 2010. PMID: 20924208 Free PMC article. Review.
References
- Barth, H., C. Schäfer, M. I. Adah, F. Zhang, R. J. Linhardt, H. Toyoda, A. Kinoshita-Toyoda, T. Toida, T. H. Van Kuppevelt, E. Depla, F. Von Weizsacker, H. E. Blum, and T. F. Baumert. 2003. Cellular binding of hepatitis C virus envelope glycoprotein E2 requires cell surface heparan sulfate. J. Biol. Chem. 278:41003-41012. - PubMed
- Bartosch, B., A. Vitelli, C. Granier, C. Goujon, J. Dubuisson, S. Pascale, E. Scarselli, R. Cortese, A. Nicosia, and F.-L. Cosset. 2003. Cell entry of hepatitis C virus requires a set of co-receptors that include the CD81 tetraspanin and the SR-B1 scavenger receptor. J. Biol. Chem. 278:41624-41630. - PubMed
- Borrow, P., and M. B. Oldstone. 1994. Mechanism of lymphocytic choriomeningitis virus entry into cells. Virology 198:1-9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical