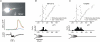Modulation of calcium wave propagation in the dendrites and to the soma of rat hippocampal pyramidal neurons - PubMed (original) (raw)
Modulation of calcium wave propagation in the dendrites and to the soma of rat hippocampal pyramidal neurons
Shigeo Watanabe et al. J Physiol. 2006.
Abstract
Repetitive synaptic stimulation in the stratum radiatum (SR) evokes large amplitude Ca2+ waves in the thick apical dendrites of hippocampal CA1 pyramidal neurons. These waves are initiated by activation of metabotropic glutamate receptors (mGluRs), which mobilize inositol-1,4,5-trisphospate (IP3) and release Ca2+ from intracellular stores. We explored mechanisms that modulate the spatial properties of these waves. Higher stimulus current evoked waves of increasing spatial extent. Most waves did not propagate through the soma; the majority stopped close to the junction of the soma and apical dendrite. Pairing strong stimulation with one electrode and subthreshold stimulation with another (associative activation) extended the waves distally but failed to extend waves into the cell body. Pairing synaptic stimulation with backpropagating action potentials enhanced the likelihood of wave generation but did not extend the waves to the somatic region. Priming the stores with Ca2+ entry through voltage dependent channels modulated wave properties but did not extend them past the dendrites. These results are consistent with propagation failing due to the dilution of synaptically generated IP3 as it diffuses into the large volume of the soma (impedance mismatch). Synaptically activating waves in the presence of low concentrations of carbachol, which probably increased the tonic level of IP3 throughout the cell, enhanced the extent of propagation and generated waves that invaded the soma, as long as low-affinity indicators were used to detect the [Ca2+]i changes. Consistent with this explanation direct injection of IP3 into the soma promoted wave propagation into this region. Ca2+ waves that propagated through the cell body were interesting because they did not fill the volume of the soma, but passed through the centre, often with large amplitude. These waves may be particularly effective in activating gene expression and protein synthesis.
Figures
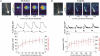
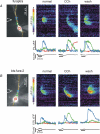
Figure 1. Synaptic stimulation at increasing intensities evokes Ca2+ waves that extend over increasing lengths of the apical dendrite
A, cell image showing a pyramidal neuron filled with 150 μ
m
bis-fura-2. The patch electrode is on the soma and the position of the stimulating electrode is shown with a dotted arrow. The set of pixels through the soma and dendrite indicate the position of the ‘line scan’ in the other panels. The box indicates the ROI (region of interest) at a branch point from where the individual optical traces were recorded. The series of panels show the fluorescence changes and electrical responses to a series of tetanic stimuli (100 Hz for 0.5 s) at increasing intensity. The threshold current was 70 μA and increments above that value are indicated below the pseudocolour images. The optical, electrical and ‘line scan’ panels all have the same time scale. At each intensity the wave initiated at a location close to the branch point covered by the ROI, as determined by the location of the earliest fluorescence change. At this location the amplitude jumped to an approximately steady level for intensities above 5 μA above threshold. However, the extent of the wave, indicated by the region of fluorescence change in the ‘line scans’, increased with higher intensities. The average amplitude and spatial extent of the waves as a function of the stimulus intensity are plotted below. Plots are referenced to the threshold current since the threshold was different for different cells. The large error bars reflect the variation from cell to cell; the plots for individual cells showed smooth increments. B, similar display and plots for five neurons filled with 300 μ
m
furaptra. The amplitude and spatial extent of the waves gradually increased with stimulus intensity. The striping in the images is due to the fact that not all pixels were positioned over the centre of the dendritic processes.
Figure 2. Most synaptically activated Ca2+ waves stop near the soma–dendrite boundary
A, [Ca2+]i changes recorded at three locations near the soma in a pyramidal neuron in response to tetanic stimulation at 100 Hz for 0.5 s. At the two dendritic locations (red and green boxes) the Ca2+ release signal was large, with almost 40% change in bis-fura-2 fluorescence. However, at the somatic location (blue box) the change was much smaller. The wave clearly failed at a location between the red and blue boxes, where the dendrite joins the soma. B, distribution of stop locations of synaptically activated propagating Ca2+ waves when bis-fura-2 (150 μ
m
) was used as the calcium indicator. Stop location was defined as the point where the amplitude of the wave fell to 30% of the peak amplitude. For each cell the stop location and the position of the stimulating electrode are shown. Histogram binning of these locations is shown below against a canonical pyramidal neuron with cell body length of 25 μm. Only a few waves propagated into the centre of the soma. C, the same analysis for experiments where furaptra (300 μ
m
) was used as the indicator. A similar distribution of stop locations was found.
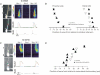
Figure 3. Associative extension of Ca2+ wave propagation
A, left, a pyramidal neuron filled with 300 μ
m
furaptra from the patch electrode on the soma. The positions of two glass stimulating electrodes are indicated in the top image, which also show the pixels underlying the line scans. The bottom image is the same cell with ROIs. Right, the electrical and optical responses when electrode 1 was stimulated (100 Hz for 0.5 s at 70 μA), electrode 2 was stimulated at the same rate at 115 μA or when both electrodes were stimulated together at those intensities (70 and 115 μA). When only electrode 1 was stimulated the line scan image shows that wave propagation extended from the soma to about 75 μm from the soma. Stimulation of electrode 2 caused no detectable [Ca2+]i change in the cell. Stimulation with both electrodes (offset by 5 ms to avoid synergistic activation of presynaptic fibres) evoked a wave that extended about an additional 25 μm into the dendrites. Only this protocol evoked a [Ca2+]i increase at the distal ROI (green box and trace). The electrical, optical, and line scan traces all have the same time scale. B, summary of results for five cells. The limits of wave propagation in the distal and proximal directions relative to the initiation site are shown. Associative stimulation distal to the stimulating electrode extended the wave front distally in all cells but had no effect on propagation in the proximal direction. The limit of wave propagation was defined as the point where the amplitude fell to 30% of the peak amplitude. C, failure to associatively extend wave propagation into the soma. At all tested intensities stimulation by electrode 2, placed close to the soma, failed to evoke a [Ca2+]i increase in the cell and failed to extend the wave generated by stimulation of electrode 1. Note the hyperpolarizing electrical response to stimulation by electrode 2, in contrast to the depolarizing response to the electrode placed in the dendritic region. D, summary of results for eight cells. In five cells the wave front extended proximally but in no case did the wave propagate through the soma.
Figure 4. Priming of stores extends the range of wave propagation but not into the soma
A, the image shows a bis-fura-2 filled pyramidal neuron with marked pixels for the line scans. Each panel shows the spatial and temporal extent of a Ca2+ wave stimulated with identical protocols (100 Hz for 0.5 s, indicated by a bar under the first image) every 2 min. Before certain trials (indicated by ‘Primed’) intrasomatically evoked spikes were evoked at 4 Hz during the interval between trials; for the other trials there was no stimulation. The time of each trial is indicated below each image. The pseudocolour images are scaled to the peak amplitude observed during the 18 trials (33%). In general, the spatial extent, indicated by the vertical limits of the coloured regions, was larger following priming. However, none of the waves extended into the soma (the boundary between the soma and dendrites is indicated by dotted lines under the images). B, distribution of the effects of priming or no priming on wave extent in 26 trials in 9 cells. Trials were divided into two categories: those following priming (left panel) and those without priming (right panel). For example, we included the change between 9 and 11 min in the experiment in A in the left panel in B and the change between 13 and 15 min in the right panel. We did not include all-or-none changes like the event between 15 and 17 min or the one between 17 and 19 min. Wave extent was compared to the previous trial. In general, priming increased the spatial extent of the waves.
Figure 5. Synaptic stimulation and action potentials together do not promote wave propagation into the soma
A, synaptic stimulation (100 Hz for 1 s) followed by 10 intrasomatically evoked spikes at 30 ms intervals. The spikes triggered the wave but the wave did not enter the soma. The [Ca2+]i increases in the soma peaked at the time of the last action potential suggesting that they were due to Ca2+ entry through voltage-dependent channels. B, a similar experiment using the indicator furaptra where a Ca2+ wave was first generated with a subthreshold tetanus (100 Hz for 0.5 s) and then generated with the same tetanus but with a burst of intrasomatically evoked action potentials. In both cases the wave had about the same spatial extent in the dendrites. The pseudocolour images were filtered with a five point interpolation in the spatial dimension.
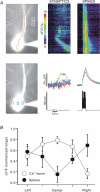
Figure 6. Extension of Ca2+ wave propagation into and through the soma by carbachol
A, wave extension monitored with 300 μ
m
furaptra in the pipette. The left panel shows the furaptra-filled pyramidal neuron with the position of the stimulating electrode, ROIs and pixels for ‘line scan’ display. Following tetanic stimulation (100 Hz for 0.5 s) in normal ACSF a Ca2+ wave was generated (middle panel) that spread in a region within the apical dendrites and did not invade the soma. When 10 μ
m
CCh was added to the ACSF (middle panel) the same stimulation evoked a wave that entered and passed through the soma. After washout a restricted wave was again recorded (right panel). B, similar experiment using 150 μ
m
bis-fura-2 as the Ca2+ indicator. In this case the wave in 10 μ
m
CCh spread over a larger region of the dendrites than in normal ACSF but did not pass through the soma.
Figure 7. Injection of IP3 into the soma promotes wave propagation into this region
A, a cell patched with 100 μ
m
IP3 and 150 μ
m
bis-fura-2 in the pipette. Following tetanic stimulation (100 Hz for 0.5 s) a Ca2+ wave was evoked that spread into the cell body. B, a similar experiment in another cell. In this case the synaptically stimulated wave was followed by a series of [Ca2+]i oscillations that were largest in the somatic region near the base of the apical dendrite. Note the slower time scale of this part of the figure. In both experiments the cell was hyperpolarized to −95 mV with current injection to prevent synaptically evoked action potentials.
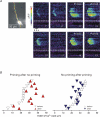
Figure 8. Wave propagation through the soma evokes [Ca2+]i increases with a different spatial distribution than [Ca2+]i increases evoked by intrasomatically stimulated action potentials
A, left panels show image of bis-fura-2 filled pyramidal neuron with positions of stimulating electrode, ROIs and line scan pixels indicated. In response to tetanic synaptic stimulation (100 Hz for 0.5 s) a Ca2+ wave was initiated in the dendrites that propagated through the soma. The optical traces show that the [Ca2+]i increase was largest in the centre with little increase on the sides. In response to spikes evoked intrasomatically, [Ca2+]i increases were generated essentially at the same time at all locations, corresponding to Ca2+ entry evoked by the backpropagating action potentials. The optical traces show that the [Ca2+]i increases were similar at all three locations. B, comparison of the relative Δ_F_/F values at different locations in the soma following either the synaptic generation of a Ca2+ wave (n = 7) or intrasomatic stimulation of spikes (n = 5). Values are normalized between the highest and lowest values in each cell to emphasize the difference between the two kinds of events.
Similar articles
- Inositol 1,4,5-trisphosphate (IP3)-mediated Ca2+ release evoked by metabotropic agonists and backpropagating action potentials in hippocampal CA1 pyramidal neurons.
Nakamura T, Nakamura K, Lasser-Ross N, Barbara JG, Sandler VM, Ross WN. Nakamura T, et al. J Neurosci. 2000 Nov 15;20(22):8365-76. doi: 10.1523/JNEUROSCI.20-22-08365.2000. J Neurosci. 2000. PMID: 11069943 Free PMC article. - Synaptically activated Ca2+ waves in layer 2/3 and layer 5 rat neocortical pyramidal neurons.
Larkum ME, Watanabe S, Nakamura T, Lasser-Ross N, Ross WN. Larkum ME, et al. J Physiol. 2003 Jun 1;549(Pt 2):471-88. doi: 10.1113/jphysiol.2002.037614. Epub 2003 Apr 11. J Physiol. 2003. PMID: 12692172 Free PMC article. - Synergistic release of Ca2+ from IP3-sensitive stores evoked by synaptic activation of mGluRs paired with backpropagating action potentials.
Nakamura T, Barbara JG, Nakamura K, Ross WN. Nakamura T, et al. Neuron. 1999 Nov;24(3):727-37. doi: 10.1016/s0896-6273(00)81125-3. Neuron. 1999. PMID: 10595522 - IP3-dependent calcium-induced calcium release mediates bidirectional calcium waves in neurones: functional implications for synaptic plasticity.
Barbara JG. Barbara JG. Biochim Biophys Acta. 2002 Nov 4;1600(1-2):12-8. doi: 10.1016/s1570-9639(02)00439-9. Biochim Biophys Acta. 2002. PMID: 12445454 Review. - Ca2+ signalling in postsynaptic dendrites and spines of mammalian neurons in brain slice.
Müller W, Connor JA. Müller W, et al. J Physiol Paris. 1992;86(1-3):57-66. doi: 10.1016/s0928-4257(05)80008-7. J Physiol Paris. 1992. PMID: 1343597 Review.
Cited by
- Complicity of α-synuclein oligomer and calcium dyshomeostasis in selective neuronal vulnerability in Lewy body disease.
Yamamoto K. Yamamoto K. Arch Pharm Res. 2021 Jun;44(6):564-573. doi: 10.1007/s12272-021-01334-6. Epub 2021 Jun 10. Arch Pharm Res. 2021. PMID: 34114191 Free PMC article. Review. - Synaptic activity induces dramatic changes in the geometry of the cell nucleus: interplay between nuclear structure, histone H3 phosphorylation, and nuclear calcium signaling.
Wittmann M, Queisser G, Eder A, Wiegert JS, Bengtson CP, Hellwig A, Wittum G, Bading H. Wittmann M, et al. J Neurosci. 2009 Nov 25;29(47):14687-700. doi: 10.1523/JNEUROSCI.1160-09.2009. J Neurosci. 2009. PMID: 19940164 Free PMC article. - mGluR5-dependent modulation of dendritic excitability in CA1 pyramidal neurons mediated by enhancement of persistent Na+ currents.
Yu W, Kwon J, Sohn JW, Lee SH, Kim S, Ho WK. Yu W, et al. J Physiol. 2018 Sep;596(17):4141-4156. doi: 10.1113/JP275999. Epub 2018 Jun 28. J Physiol. 2018. PMID: 29870060 Free PMC article. - Priming of intracellular calcium stores in rat CA1 pyramidal neurons.
Hong M, Ross WN. Hong M, et al. J Physiol. 2007 Oct 1;584(Pt 1):75-87. doi: 10.1113/jphysiol.2007.137661. Epub 2007 Aug 9. J Physiol. 2007. PMID: 17690146 Free PMC article. - Deviant ryanodine receptor-mediated calcium release resets synaptic homeostasis in presymptomatic 3xTg-AD mice.
Chakroborty S, Goussakov I, Miller MB, Stutzmann GE. Chakroborty S, et al. J Neurosci. 2009 Jul 29;29(30):9458-70. doi: 10.1523/JNEUROSCI.2047-09.2009. J Neurosci. 2009. PMID: 19641109 Free PMC article.
References
- Alger BE. Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol. 2002;68:247–286. - PubMed
- Allbritton NL, Meyer T, Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science. 1992;258:1812–1815. - PubMed
- Augustine GJ, Santamaria F, Tanaka K. Local calcium signaling in neurons. Neuron. 2003;40:331–346. - PubMed
- Bannister NJ, Larkman AU. Dendritic morphology of CA1 pyramidal neurones from the rat hippocampus. II. Spine distributions. J Comp Neurol. 1995;360:161–171. - PubMed
- Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous