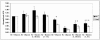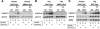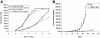5'-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments - PubMed (original) (raw)
5'-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments
Keith R Laderoute et al. Mol Cell Biol. 2006 Jul.
Abstract
Low oxygen gradients (hypoxia and anoxia) are important determinants of pathological conditions under which the tissue blood supply is deficient or defective, such as in solid tumors. We have been investigating the relationship between the activation of hypoxia-inducible factor 1 (HIF-1), the primary transcriptional regulator of the mammalian response to hypoxia, and 5'-AMP-activated protein kinase (AMPK), another regulatory system important for controlling cellular energy metabolism. In the present study, we used mouse embryo fibroblasts nullizygous for HIF-1alpha or AMPK expression to show that AMPK is rapidly activated in vitro by both physiological and pathophysiological low-oxygen conditions, independently of HIF-1 activity. These findings imply that HIF-1 and AMPK are components of a concerted cellular response to maintain energy homeostasis in low-oxygen or ischemic-tissue microenvironments. Finally, we used transformed derivatives of wild-type and HIF-1alpha- or AMPKalpha-null mouse embryo fibroblasts to determine whether AMPK is activated in vivo. We obtained evidence that AMPK is activated in authentic hypoxic tumor microenvironments and that this activity overlaps with regions of hypoxia detected by a chemical probe. We also showed that AMPK is important for the growth of this tumor model.
Figures
FIG. 1.
Total cellular ATP levels in WT and HIF-1α-null cells exposed to prolonged hypoxia in the presence and absence of glucose. ATP levels were measured in whole-cell lysates of WT and HIF-1α-null MEFs exposed to normoxia (Air + Glucose) or hypoxia (Hx) in complete (+ Glucose) or glucose-free (− Glucose) medium for 8 h. In glucose reconstitution experiments, degassed glucose solution was added to a final concentration of 4.5 g/liter at either 30 min (+, 30 min) or 1 h (+, 1 h) before cell lysis. Data were normalized to measurements of total ATP in normoxic cells in complete medium (Air + Glucose). Error bars, standard deviations from three independent experiments. Statistical comparisons were made between WT and HIF-1α-null cells for each condition shown (only the differences indicated by one or more asterisks are statistically significant). *, P ≤ 0.02, Hx + Glucose; **, P ≤ 0.04, Hx − Glucose (+, 30 min); ***, P ≤ 0.02, Hx − Glucose (+, 1 h). The addition of glucose to both WT and HIF-1α-null cells exposed to hypoxia and glucose deprivation increased total cellular ATP levels by 1 h after the addition [WT cells, P ≤ 0.005; HIF-1α-null cells, P ≤ 0.005; Hx − Glucose compared with Hx − Glucose (+, 1 h)].
FIG. 2.
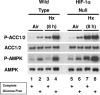
Activation of AMPK in WT and HIF-1α-null cells exposed to prolonged hypoxia in the presence and absence of glucose. Immunoblot assays of total protein from WT and HIF-1α-null MEFs lysed following exposure to normoxia or hypoxia in complete or glucose-free medium for 8 h. Replicate blots were probed for the relative levels of P-ACC1/2, total ACC1/2, P-AMPK, or total AMPK. For details, see Materials and Methods. These results are representative of at least three independent experiments.
FIG. 3.
Requirement for AMPK to phosphorylate ACC1/2 in cells exposed to prolonged hypoxia in the presence and absence of glucose. (A) Immunoblot assays of total protein from primary WT and AMPK-null MEFs lysed following exposure to normoxia or hypoxia in complete or glucose-free medium for 8 h. (B) Immunoblot assays of total protein from immortalized WT and AMPK-null MEFs lysed following exposure to normoxia or hypoxia in complete or glucose-free medium for 8 h. (C) Immunoblot assays of total protein from primary and immortalized WT and AMPK-null MEFs exposed to these conditions. Replicate blots were probed for the relative levels of P-ACC1/2, total ACC1/2, and total AMPK. Immortalized MEFs were used for all other studies involving genetic knockouts of the AMPKα subunits. TAg, simian virus 40 large T antigen.
FIG. 4.
Time courses of ACC1/2 phosphorylation in WT, HIF-1α-null, and AMPKα-null cells exposed to hypoxia in complete medium. (A) Immunoblot assays of total protein from WT and HIF-1α-null MEFs harvested following exposure to normoxia or hypoxia for the indicated times. (B) Immunoblot assays of total protein from WT and AMPKα-null MEFs harvested following exposure to normoxia or hypoxia for the indicated times. Replicate blots were probed for the relative levels of P-ACC1/2 and total ACC1/2.
FIG. 5.
ACC1/2 phosphorylation in AMPKα1-null and AMPKα2-null cells exposed to hypoxia in the presence and absence of glucose. (A) Immunoblot assays of total protein from WT, AMPKα1-null, and AMPKα2-null MEFs lysed following exposure to normoxia or hypoxia in complete or glucose-free medium for 8 h. (B) Immunoblot assays of total protein from AMPKα1-null and AMPKα2-null MEFs lysed following exposure to normoxia or hypoxia for the indicated times. Replicate blots were probed for the relative levels of P-ACC1/2 and total ACC1/2. These results are representative of two independent experiments.
FIG. 6.
ACC1/2 phosphorylation in WT and AMPKα-null cells exposed to physiological hypoxia. Immunoblot assays of total protein from WT and AMPKα-null MEFs harvested following exposure to normoxia or hypoxia (pO2 = 1%) in complete medium for the indicated times. Replicate blots were probed for the relative levels of P-ACC1/2, total ACC1/2, and HIF-1α.
FIG. 7.
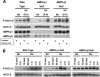
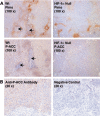
Phosphorylation of ACC1/2 in hypoxic regions in experimental tumors prepared from H-Ras-transformed MEFs. (A) Representative images of immunostaining for pimonidazole (Pimo) binding and ACC1/2 phosphorylation (P-ACC) on 4-μm-thick contiguous sections of paraffin-embedded tissue blocks from WT and HIF-1α-null MEF xenografts. Arrows indicate necrotic regions in the sections. (B) Images of immunostaining for ACC1/2 phosphorylation on 4-μm-thick contiguous sections of a paraffin-embedded block from a WT MEF xenograft. The image on the right, which was obtained without using the primary anti-P-ACC1/2 antibody, is a negative control for the immunological detection of ACC1/2 phosphorylation.
FIG. 8.
Effect of loss of AMPK activity on the proliferation of H-Ras-transformed MEFs. (A) In vitro proliferation of WT (closed symbols) and AMPKα-null (open symbols) MEFs determined by an Alamar Blue assay. Cells/well refer to 96-well plates (triangles, 2,000 cells/well; squares, 250 cells/well). (B) Growth of tumor xenografts prepared from the WT and AMPKα-null MEFs used for the in vitro proliferation assay. There were eight mice in each of the two groups corresponding to the WT (closed symbols) and AMPKα-null (AMPKa, open symbols) tumor genotypes (for details, see Materials and Methods). Error bars are standard deviations.
Similar articles
- AMP-activated protein kinase activity is required for vanadate-induced hypoxia-inducible factor 1alpha expression in DU145 cells.
Hwang JT, Lee M, Jung SN, Lee HJ, Kang I, Kim SS, Ha J. Hwang JT, et al. Carcinogenesis. 2004 Dec;25(12):2497-507. doi: 10.1093/carcin/bgh253. Epub 2004 Aug 5. Carcinogenesis. 2004. PMID: 15297373 - AMP-activated protein kinase activity is critical for hypoxia-inducible factor-1 transcriptional activity and its target gene expression under hypoxic conditions in DU145 cells.
Lee M, Hwang JT, Lee HJ, Jung SN, Kang I, Chi SG, Kim SS, Ha J. Lee M, et al. J Biol Chem. 2003 Oct 10;278(41):39653-61. doi: 10.1074/jbc.M306104200. Epub 2003 Aug 4. J Biol Chem. 2003. PMID: 12900407 Retracted. - Glucose deprivation increases mRNA stability of vascular endothelial growth factor through activation of AMP-activated protein kinase in DU145 prostate carcinoma.
Yun H, Lee M, Kim SS, Ha J. Yun H, et al. J Biol Chem. 2005 Mar 18;280(11):9963-72. doi: 10.1074/jbc.M412994200. Epub 2005 Jan 7. J Biol Chem. 2005. PMID: 15640157 Retracted. - AMP-activated protein kinase couples mitochondrial inhibition by hypoxia to cell-specific Ca2+ signalling mechanisms in oxygen-sensing cells.
Evans AM, Hardie DG, Galione A, Peers C, Kumar P, Wyatt CN. Evans AM, et al. Novartis Found Symp. 2006;272:234-52; discussion 252-8, 274-9. Novartis Found Symp. 2006. PMID: 16686439 Review. - [AMP-activated protein kinase: how a mistake in energy gauge causes glycogen storage].
Ofir M, Hochhauser E, Vidne BA, Freimark D, Arad M. Ofir M, et al. Harefuah. 2007 Oct;146(10):770-5, 813-4. Harefuah. 2007. PMID: 17990392 Review. Hebrew.
Cited by
- Effect of metformin on the proliferation, migration, and MMP-2 and -9 expression of human umbilical vein endothelial cells.
Esfahanian N, Shakiba Y, Nikbin B, Soraya H, Maleki-Dizaji N, Ghazi-Khansari M, Garjani A. Esfahanian N, et al. Mol Med Rep. 2012 Apr;5(4):1068-74. doi: 10.3892/mmr.2012.753. Epub 2012 Jan 12. Mol Med Rep. 2012. PMID: 22246099 Free PMC article. - AMP-activated protein kinase is required for the macropinocytic internalization of ebolavirus.
Kondratowicz AS, Hunt CL, Davey RA, Cherry S, Maury WJ. Kondratowicz AS, et al. J Virol. 2013 Jan;87(2):746-55. doi: 10.1128/JVI.01634-12. Epub 2012 Oct 31. J Virol. 2013. PMID: 23115293 Free PMC article. - The double-edged sword of AMPK signaling in cancer and its therapeutic implications.
Jeon SM, Hay N. Jeon SM, et al. Arch Pharm Res. 2015 Mar;38(3):346-57. doi: 10.1007/s12272-015-0549-z. Epub 2015 Jan 10. Arch Pharm Res. 2015. PMID: 25575627 Free PMC article. Review. - AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress.
Jeon SM, Chandel NS, Hay N. Jeon SM, et al. Nature. 2012 May 9;485(7400):661-5. doi: 10.1038/nature11066. Nature. 2012. PMID: 22660331 Free PMC article. - Compartmentalized AMPK signaling illuminated by genetically encoded molecular sensors and actuators.
Miyamoto T, Rho E, Sample V, Akano H, Magari M, Ueno T, Gorshkov K, Chen M, Tokumitsu H, Zhang J, Inoue T. Miyamoto T, et al. Cell Rep. 2015 Apr 28;11(4):657-70. doi: 10.1016/j.celrep.2015.03.057. Epub 2015 Apr 16. Cell Rep. 2015. PMID: 25892241 Free PMC article.
References
- Airley, R. E., J. Loncaster, J. A. Raleigh, A. L. Harris, S. E. Davidson, R. D. Hunter, C. M. West, and I. J. Stratford. 2003. GLUT-1 and CAIX as intrinsic markers of hypoxia in carcinoma of the cervix: relationship to pimonidazole binding. Int. J. Cancer 104:85-91. - PubMed
- Almeida, A., S. Moncada, and J. P. Bolanos. 2004. Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat. Cell Biol. 6:45-51. - PubMed
- Arcasoy, M. O., K. Amin, S. C. Chou, Z. A. Haroon, M. Varia, and J. A. Raleigh. 2005. Erythropoietin and erythropoietin receptor expression in head and neck cancer: relationship to tumor hypoxia. Clin. Cancer Res. 11:20-27. - PubMed
- Barabasi, A. L., and Z. N. Oltvai. 2004. Network biology: understanding the cell's functional organization. Nat. Rev. Genet. 5:101-113. - PubMed
- Beauloye, C., A. S. Marsin, L. Bertrand, U. Krause, D. G. Hardie, J. L. Vanoverschelde, and L. Hue. 2001. Insulin antagonizes AMP-activated protein kinase activation by ischemia or anoxia in rat hearts, without affecting total adenine nucleotides. FEBS Lett. 505:348-352. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases