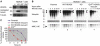HDAC6-p97/VCP controlled polyubiquitin chain turnover - PubMed (original) (raw)
HDAC6-p97/VCP controlled polyubiquitin chain turnover
Cyril Boyault et al. EMBO J. 2006.
Abstract
HDAC6 is a unique cytoplasmic deacetylase capable of interacting with ubiquitin. Using a combination of biophysical, biochemical and biological approaches, we have characterized the ubiquitin-binding domain of HDAC6, named ZnF-UBP, and investigated its biological functions. These studies show that the three Zn ion-containing HDAC6 ZnF-UBP domain presents the highest known affinity for ubiquitin monomers and mediates the ability of HDAC6 to negatively control the cellular polyubiquitin chain turnover. We further show that HDAC6-interacting chaperone, p97/VCP, dissociates the HDAC6-ubiquitin complexes and counteracts the ability of HDAC6 to promote the accumulation of polyubiquitinated proteins. We propose that a finely tuned balance of HDAC6 and p97/VCP concentrations determines the fate of ubiquitinated misfolded proteins: p97/VCP would promote protein degradation and ubiquitin turnover, whereas HDAC6 would favour the accumulation of ubiquitinated protein aggregates and inclusion body formation.
Figures
Figure 1
Characterization of the HDAC6 ZnF-UBP–ubiquitin interaction. (A) Schematic representation of HDAC6 functional domains. The two catalytic domains, DD1 and DD2, as well as the ubiquitin-binding domain, ZnF-UBP, of mouse HDAC6 are indicated. (B) HDAC6 ZnF-UBP forms a stable 1:1 complex with ubiquitin. Size-exclusion chromatography shows the formation of the HDAC6 ZnF-UBP/ubiquitin complex (upper panel). The lower panel shows the peak fractions analysed by SDS–PAGE. I stands for input. (C) Overlay of data (points) and fitted curves (lines) for a global analysis of equilibrium sedimentation data. The scans taken at multiple loading concentrations and multiple speeds are fit to single-component models: ubiquitin (I); HDAC6 ZnF-UBP (II); HDAC6 ZnF-UBP/ubiquitin complex (III). (D) ITC profile for the binding of ubiquitin to HDAC6 ZnF-UBP. Data were fitted to a one-site model. Values obtained for the binding were as follows: K_D=60 nM; Δ_H_=−17.9 kcal/mol; −_T*Δ_S_=8.3 kcal/mol. The stoichiometry of binding was 1:1.
Figure 2
The HDAC6 ZnF-UBP domain contains three Zn atoms critical for ubiquitin binding. (A) EXAFS analysis of the HDAC6 ZnF-UBP domain was performed. Left panel: EXAFS spectrum; right panel: Fourier transformation. Experimental and theoretical spectra are represented as solid and dashed lines, respectively. The refinement resulted in an average Zn environment with 1.1 (±0.3) imidazole unit at 2.01(1) Å and 2.9 (±0.3) sulphur ligands at 2.31(1) Å. The Debye–Waller factors (2σ2=0.006(1) Å2) and the energy shift Δ_E_F=−9(1) eV with EF=9660 eV were refined jointly for both shells. (B) Alignment of ZnF-UBP domains from the indicated proteins based on the crystal structure of the ZnF-UBP domain of USP5 (Reyes-Turcu et al, 2006). Conserved cysteine and histidine residues are highlighted. These residues cluster in positions Zn1, Zn2 and Zn3. In insertions, the number of omitted residues is marked in bold. SwissProt Database entries shown are as follows: HDAC6 (Mus musculus; SW: Q9Z2V5), USP5 (human; SW: P45974), UBP3 (human; SW: Q9Y6I4), UBP8 (Saccharomyces cerevisiae; SW: P50102), UBP13 (human; SW: Q92995), UBP14 (S. cerevisiae; SW: P38237), UBP16 (human; SW: Q9Y5T5), UBP20 (human; SW: Q9Y2K6), UBP22 (human; SW: Q9UPT9), UBP33 (human; SW: Q8TEY7), UBP44 (human; SW: Q9H0E7), USP45 (human; SW: Q9BRU1), UBP49 (human; SW: Q70CQ1) and UBP51 (human; SW: Q70EK9). (C) Ribbon representation of the USP5 ZnF-UBP domain. Zn1 corresponds to the Zn site observed in the crystal structure of USP5. Cys/His residues conserved in most ZNF-UBP domains cluster at two additional sites Zn2 and Zn3. For the ribbon diagram, USP5 residues were mutated to the corresponding Cys/His residues present in HDAC6. Asterisks indicate the predicted Zn positions. (D) The indicated cysteines and histidines in the ZnF-UBP domain of HDAC6 were replaced by alanine and the corresponding coding sequence (1047–1121 region of HDAC6) cloned in an expression vector. These constructs were then used to obtain 35S-labelled proteins. A ubiquitin pull-down experiment was performed to evaluate the ubiquitin-binding activity of the _in vitro_-translated proteins. The input panel show 20% of the material used in the pull-down assays. Proteins retained on the ubiquitin beads are shown in the lower panel.
Figure 3
HDAC6 polyubiquitin masking effect. A 1 μM portion of purified penta-ubiquitin chains was pre-incubated with 6 μM of purified HDAC6 or 6 μM of purified RPN10 (see also Figure 5A). (A) Equivalent amounts of purified UBPY were then added and the reactions stopped at the indicated times, and the ubiquitin cleavage was visualized using an anti-ubiquitin antibody (ubiquitin panel). Anti-GST and anti-Flag antibodies were used to show the amounts of GST-UBPY and Flag-tagged proteins in each condition (indicated). (B) Depolymerization of polyubiquitin chain into ubiquitin monomers was monitored by Western blot and quantified and shown. 100% represents the amount of polyubiquitin at time 0.
Figure 4
HDAC6 controls the turnover of polyubiquitin chains. (A) 3T3 cells were derived from parental HDAC6+/+ or HDAC6−/− mice (KO cells). KO cells were used to establish cell lines expressing either wild-type HDAC6 (WT) or a non-ubiquitin-binding mutant of HDAC6 (ZnFm). Extracts from these cells were used to monitor HDAC6 expression (HDAC6 panel). The amounts of acetylated tubulin and tubulin in the extracts were also visualized by appropriate antibodies (AcTubulin and Tubulin panels, respectively). (B) The accumulation of heavily ubiquitinated proteins was induced after treating cells described in panel A by the proteasome inhibitor MG132 (10 μM) for 16 h. At time _t_=0, cells were washed and cultured in a fresh drug-free medium for the indicated times. Extracts were prepared, and ubiquitinated proteins were visualized by an anti-ubiquitin antibody. An antibody raised against an MHC class I heavy chain was also used to monitor its abundance in the same experiments (MHC HC panel). (C) The intensity of signals from ubiquitinated proteins in the indicated regions (brackets) was measured using the densitometric signals from the Western blots (right panel) and the values were normalized with respect to that of tubulin and plotted as a % of time 0.
Figure 5
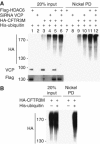
HDAC6–ubiquitin interaction hinders ubiquitin recognition by the ubiquitin-binding 19S protein, RPN10, and p97/VCP reverts the effect of HDAC6. (A) Cos cells were massively transfected with expression vectors encoding Flag-tagged HDAC6, p97/VCP and RPN10. Flag-tagged proteins were immunoprecipitated and eluted using an excess of Flag peptides and then concentrated. (B) His-tagged K48 5+1 ubiquitin chains were immobilized on Ni beads and incubated with an excess of purified HDAC6, RPN10 and p97/VCP. In each experiment involving HDAC6, an excess of HDAC6 was preincubated with the ubiquitin chains and, after removal of the unbound proteins, the beads were incubated with purified p97/VCP and/or RPN10 (indicated by +). After washing the beads, the proteins retained on ubiquitin were denatured and detected by the anti-Flag antibody. The experiments were performed in parallel in the absence and presence of ATP (indicated). The left panel shows the input materials. (C) The supernatant corresponding to the experiment shown in lane 13 was used to immunoprecipitate HDAC6 (using an anti-HDAC6 antibody) and the immunoprecipitated proteins were analysed by probing the blot with an anti-Flag antibody.
Figure 6
HDAC6–p97/VCP controlled protein polyubiquitination. (A) Cos cells were transfected with control siRNA or p97/VCP-specific siRNA and also with the indicated expression vectors. At 48 h post-transfection, the cells were lysed. A fraction of the lysate was kept aside (input panel) and the rest was used to capture ubiquitinated proteins on Ni beads. Input and captured materials were analysed by an anti-HA antibody. p97/VCP and Flag-HDAC6 were revealed in the input materials, and are shown at the bottom of the input panel. (B) CFTR3M was expressed with and without 6 His-ubiquitin (+ and −, respectively), and input and captured materials are shown.
Figure 7
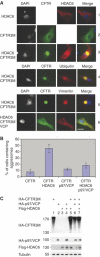
p97/VCP expression reduces the efficiency of HDAC6-dependent aggresome formation. (A) Cos cells were transfected with vectors expressing CFTR3M alone or together with HDAC6, p97/VCP or both. Cells were fixed 24 h post-transfection and different proteins were visualized. The upper panel shows the detection of HDAC6 (1), CFTR3M (2) or both (3). In the latter case, different combinations of immunodetection were used. CFTR3M was co-detected with HDAC6 (3), ubiquitin (4) or vimentin (5). The lower panels (5 and 6) show the cellular distribution of CFTR3M and vimentin in HDAC6-expressing cells, in the absence (5) or presence (6) of p97/VCP expression. (B) The % of cells forming CFTR3M aggresomes in each condition was determined as judged by cells containing vimentin cages and represented as a histogram. (C) Extracts were prepared from cells transfected in the same experiments as described above and CFTR3M, HDAC6 and p97/VCP were visualized (indicated).
Similar articles
- The heavy metal cadmium induces valosin-containing protein (VCP)-mediated aggresome formation.
Song C, Xiao Z, Nagashima K, Li CC, Lockett SJ, Dai RM, Cho EH, Conrads TP, Veenstra TD, Colburn NH, Wang Q, Wang JM. Song C, et al. Toxicol Appl Pharmacol. 2008 May 1;228(3):351-63. doi: 10.1016/j.taap.2007.12.026. Epub 2008 Jan 8. Toxicol Appl Pharmacol. 2008. PMID: 18261755 Free PMC article. - HDAC6-ubiquitin interaction controls the duration of HSF1 activation after heat shock.
Pernet L, Faure V, Gilquin B, Dufour-Guérin S, Khochbin S, Vourc'h C. Pernet L, et al. Mol Biol Cell. 2014 Dec 15;25(25):4187-94. doi: 10.1091/mbc.E14-06-1032. Epub 2014 Oct 8. Mol Biol Cell. 2014. PMID: 25298398 Free PMC article. - The Ankrd13 Family of Ubiquitin-interacting Motif-bearing Proteins Regulates Valosin-containing Protein/p97 Protein-mediated Lysosomal Trafficking of Caveolin 1.
Burana D, Yoshihara H, Tanno H, Yamamoto A, Saeki Y, Tanaka K, Komada M. Burana D, et al. J Biol Chem. 2016 Mar 18;291(12):6218-31. doi: 10.1074/jbc.M115.710707. Epub 2016 Jan 21. J Biol Chem. 2016. PMID: 26797118 Free PMC article. - New insights into the non-enzymatic function of HDAC6.
Zhu Y, Feng M, Wang B, Zheng Y, Jiang D, Zhao L, Mamun MAA, Kang H, Nie H, Zhang X, Guo N, Qin S, Wang N, Liu H, Gao Y. Zhu Y, et al. Biomed Pharmacother. 2023 May;161:114438. doi: 10.1016/j.biopha.2023.114438. Epub 2023 Mar 9. Biomed Pharmacother. 2023. PMID: 37002569 Review. - Valosin-Containing Protein (VCP)/p97: A Prognostic Biomarker and Therapeutic Target in Cancer.
Costantini S, Capone F, Polo A, Bagnara P, Budillon A. Costantini S, et al. Int J Mol Sci. 2021 Sep 21;22(18):10177. doi: 10.3390/ijms221810177. Int J Mol Sci. 2021. PMID: 34576340 Free PMC article. Review.
Cited by
- An old dog learns new tricks: a novel function for Cdc20-APC in dendrite morphogenesis in neurons.
Puram SV, Kim AH, Bonni A. Puram SV, et al. Cell Cycle. 2010 Feb 1;9(3):482-5. doi: 10.4161/cc.9.3.10558. Cell Cycle. 2010. PMID: 20195072 Free PMC article. - Stress granule: A promising target for cancer treatment.
Gao X, Jiang L, Gong Y, Chen X, Ying M, Zhu H, He Q, Yang B, Cao J. Gao X, et al. Br J Pharmacol. 2019 Dec;176(23):4421-4433. doi: 10.1111/bph.14790. Epub 2019 Nov 8. Br J Pharmacol. 2019. PMID: 31301065 Free PMC article. Review. - Inhibition of autophagy, lysosome and VCP function impairs stress granule assembly.
Seguin SJ, Morelli FF, Vinet J, Amore D, De Biasi S, Poletti A, Rubinsztein DC, Carra S. Seguin SJ, et al. Cell Death Differ. 2014 Dec;21(12):1838-51. doi: 10.1038/cdd.2014.103. Epub 2014 Jul 18. Cell Death Differ. 2014. PMID: 25034784 Free PMC article. - Knockdown of transactive response DNA-binding protein (TDP-43) downregulates histone deacetylase 6.
Fiesel FC, Voigt A, Weber SS, Van den Haute C, Waldenmaier A, Görner K, Walter M, Anderson ML, Kern JV, Rasse TM, Schmidt T, Springer W, Kirchner R, Bonin M, Neumann M, Baekelandt V, Alunni-Fabbroni M, Schulz JB, Kahle PJ. Fiesel FC, et al. EMBO J. 2010 Jan 6;29(1):209-21. doi: 10.1038/emboj.2009.324. Epub 2009 Nov 12. EMBO J. 2010. PMID: 19910924 Free PMC article. - Post-translational modifications of tubulin: pathways to functional diversity of microtubules.
Song Y, Brady ST. Song Y, et al. Trends Cell Biol. 2015 Mar;25(3):125-36. doi: 10.1016/j.tcb.2014.10.004. Epub 2014 Nov 25. Trends Cell Biol. 2015. PMID: 25468068 Free PMC article. Review.
References
- Amerik AY, Li SJ, Hochstrasser M (2000) Analysis of the deubiquitinating enzymes of the yeast Saccharomyces cerevisiae. Biol Chem 381: 981–992 - PubMed
- Bertos NR, Gilquin B, Chan GK, Yen TJ, Khochbin S, Yang XJ (2004) Role of the tetradecapeptide repeat domain of human histone deacetylase 6 in cytoplasmic retention. J Biol Chem 279: 48246–48254 - PubMed
- Destaing O, Saltel F, Gilquin B, Chabadel A, Khochbin S, Ory S, Jurdic P (2005) A novel Rho–mDia2–HDAC6 pathway controls podosome patterning through microtubule acetylation in osteoclasts. J Cell Sci 118: 2901–2911 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous