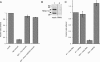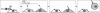An MMP liberates the Ninjurin A ectodomain to signal a loss of cell adhesion - PubMed (original) (raw)
. 2006 Jul 15;20(14):1899-910.
doi: 10.1101/gad.1426906. Epub 2006 Jun 30.
Affiliations
- PMID: 16815999
- PMCID: PMC1522090
- DOI: 10.1101/gad.1426906
An MMP liberates the Ninjurin A ectodomain to signal a loss of cell adhesion
Shuning Zhang et al. Genes Dev. 2006.
Abstract
Matrix metalloproteinases (MMPs) are important for developmental tissue remodeling and for the inflammatory response. Although the vertebrate MMP family is large and functionally redundant, the fruitfly Drosophila melanogaster has only two MMPs, both essential genes. Our previous work demonstrated that Mmp1 is required for growth of the tracheal system, and we suggested that the mutant phenotype resulted from aberrant persistence of cell adhesion to the extracellular matrix. Here we report the identification of NijA, a transmembrane protein whose vertebrate homologs regulate cell adhesion, as a two-hybrid binding partner for Mmp1. The binding of Mmp1 and NijA was confirmed by coimmunoprecipitation of endogenous proteins from flies, and the endogenous proteins were found to colocalize at the tracheal cell surface in larvae. When NijA is expressed in S2 cells, they lose adhesion to surfaces; this adhesion-loss phenotype is dependent on the expression and catalytic activity of Mmp1. Our data indicate that Mmp1 releases the N-terminal extracellular domain of NijA. This liberated ectodomain promotes the loss of cell adhesion in a cell-nonautonomous manner. We suggest that tracheal cell adhesion is regulated by a novel mechanism utilizing an MMP and a ninjurin family member.
Figures
Figure 1.
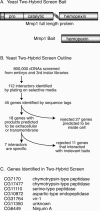
A yeast two-hybrid screen to identify proteins that bind to the Mmp1 hemopexin domain. (A) Schematic of Mmp1 domain structure in the wild-type protein and in the bait construct. The hemopexin domain of splice form 1 was used as two-hybrid bait. (ss) Signal sequence; (h) hinge domain. (B) Outline of the screen and secondary screens. (C) The seven genes identified in this screen.
Figure 2.
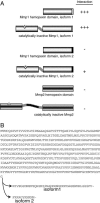
NijA protein sequence, homology, and topology. (A) Drosophila NijA amino acid sequence, from CG6449. Gray boxes indicate predicted transmembrane domains. The black box indicates the region corresponding to the antigenic peptide. NijA splice form B is shown; isoform A removes VNRT (amino acids 23–26); isoform C removes VPETDDDDNDDRPFV (amino acids 52–66). The cDNA fragment identified by two-hybrid screening encodes amino acids 43–end, consistent with either isoform A or B. (B) ClustalW alignment of human Ninjurin-1 and Ninjurin-2 and Drosophila Ninjurin A. Black boxes indicate identical residues; gray boxes indicate similar residues. Isoform B of NijA is shown, and the human splice forms are the ones reported by Araki and Milbrandt (1996, 2000). (C) Predicted NijA topology, with the termini outside the cell. (D) Phylogenetic tree relating all ninjurin domain-containing proteins identified to date. Non-insect proteins shown are rat Ninjurin-1 and Ninjurin-2, human Ninjurin-1 and Ninjurin-2, BAE34827.1 (Mus musculus), XP 853215.1 (Canis familiaris), XP 528716.1 (Pan troglodytes), XP 586318.2 (Bos taurus), XP 414328.1 (Gallus gallus), NP 001008021.1 (Xenopus tropicalis), CAG06884.1 (Tetraodon nigroviridis), XP 416382.1 (Gallus gallus), XP 854702.1 (Canis familiaris), CAF97213.1 (Tetraodon nigroviridis), XP 522310.1 (Pan troglodytes), XP 586544.2 (Bos taurus), and XP 783902.1 (Strongylocentrotus purpuratus).
Figure 3.
Specificity of the two-hybrid interaction between NijA and Mmp1. (A) The indicated bait constructs were tested for their ability to interact with a NijA prey construct. Interaction was measured by the growth of 18 replicate colonies on −leu −trp medium. (B) Comparison of the form 1 and form 2 splice forms of Mmp1.
Figure 4.
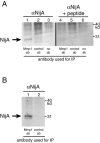
NijA antibody. S2 cells stably transformed with a copper-inducible NijA plasmid expressed a protein recognized by anti-NijA antibodies when induced. (Right side) Cell extracts probed with anti-NijA whole serum. (Left side) Cell extracts probed with anti-NijA antibodies preabsorbed against the antigenic peptide, demonstrating the specificity of the antibodies. Length of NijA induction is shown for each lane.
Figure 5.
NijA coimmunoprecipitates with Mmp1 in vivo. (A) NijA coimmunoprecipitates with catalytically inactive Mmp1. Protein extracts were made from transgenic animals expressing catalytically dead Mmp1.f1 ubiquitously (genotype tubP-GAL4/+; UAS-Mmp1.f1E225A/+). Extracts were precipitated with mixed monoclonal anti-Mmp1 antibodies (lanes 1, 4), control monoclonal antibodies (lanes 2, 5), or no primary antibodies (lanes 3, 6). Two identical blots were probed: one with anti-NijA antibodies (lanes 1–3), and one with anti-NijA antibodies preabsorbed with the competing immunogenic peptide (lanes 4–6). (B) NijA coimmunoprecipitates with endogenous wild-type Mmp1 in vivo. Protein extracts were made from wild-type animals and precipitated with mixed monoclonal anti-Mmp1 antibodies (lane 1) or control antibodies (lane 2). The blot was probed with affinity-purified anti-NijA antibodies.
Figure 6.
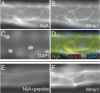
NijA and Mmp1 colocalize to cell surfaces in trachea. A–D are images of one sample; E–F are images of another sample. (A) NijA whole serum recognizes cell surfaces in tracheal dorsal trunks. (B) Mmp1 antibodies also recognize cell surfaces in dorsal trunks. (C) DAPI staining of nuclei in the same sample. (D) A merged image of the images in A–C showing the colocalization of NijA and Mmp1. (E) The cell surface staining is blocked by preabsorbing the NijA antibody with the antigenic peptide, demonstrating the specificity of the antibody in fixed tissue. (F) Mmp1 staining is unaffected by antibody preabsorption with the NijA antigenic peptide.
Figure 7.
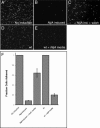
NijA conditions the medium to inhibit cell adhesion. (A–F) _NijA_-expressing cells were induced for 3 h; half were resuspended in fresh medium, and both samples were allowed to settle for 2 h on glass slides (B, C; columns 2,3 in F). Wild-type cells plated at the same density as the NijA cells were split; half were resuspended in the medium conditioned by the NijA cells, and both samples were allowed to settle on glass slides (D, E; columns 4,5 in F). (A–E) Photographs of DAPI-stained cells of the indicated genotypes, treated as described: (A) _NijA_-transformed cells, not induced to express NijA. (B) _NijA_-transformed cells induced to express NijA for 3 h. (C) _NijA_-transformed cells induced to express NijA for 3 h, then resuspended in fresh medium. (D) Wild-type cells. (E) Wild-type cells removed to _NijA_-conditioned medium. (F) Quantification of the fraction of adherent cells. The fraction of adherent cells in columns 1–3 was determined by dividing the number of adherent cells counted in each sample by the number of adherent _NijA_-uninduced cells counted in the sample shown in column 1. The fraction adhering in columns 4 and 5 was determined by dividing the number of adherent cells by the number of wild-type adherent cells in the sample shown in column 4. Thus, columns 1 and 4 are defined as 1. Error bars indicate SEM; data from four experiments are shown.
Figure 8.
Mmp1 activity is required for the _NijA_-mediated loss of adhesion. (A) S2 cells stably transformed with inducible NijA were transiently transformed with MT-GAL4 and UAS-Timp, so that the addition of copper induced the expression of Timp (Klueg et al. 2002); or the plasmid pRmHa-3.Mmp1.f1E225A, which expresses a catalytically inactive mutant of Mmp1 upon induction; or MT-GAL4 as a control. Column 1 shows the baseline adherence of uninduced control-transfected cells. Column 2 shows the adherence of control-transfected cells induced to express NijA. Column 3 shows the adherence of cells transfected and induced to express NijA and Timp. Column 4 shows the adherence of cells transfected and induced to express NijA and Mmp1.f1E225A. Adherent fractions were determined by normalizing to the number of adherent cells in the control transformed, uninduced cells (shown in column 1). Error bars indicate SEM; data from three experiments are shown. (B) Western blot with anti-Mmp1 antibodies to determine the level of Mmp1 in dsRNA experiments. (Lane 1) Untreated cells. (Lane 2) Cells treated with dsRNA against Mmp1. (C) S2 cells stably transformed with inducible NijA were treated with dsRNA against Mmp1, or mock-treated with no RNA. Column 1 shows the fraction of adherent wild-type cells mock treated and put under induction conditions. Column 2 shows the fraction of adherent wild-type cells treated with dsRNA against Mmp1. Column 3 shows the fraction of adherent mock-treated _NijA_-expressing cells. Column 4 shows the fraction of adherent _NijA_-expressing cells, treated with dsRNA against Mmp1. Adherent fractions were determined by normalizing to the number of wild-type mock-treated adherent cells (column 1). Error bars indicate SEM; data from four experiments are shown.
Figure 9.
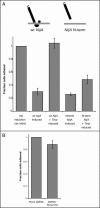
The secreted N-terminal fragment of NijA signals the loss of cell adhesion in an MMP-independent manner. (A) Wild-type S2 cells were transiently transfected with the NijA constructs shown, either with or without the MT-GAL4 and UAS-Timp plasmids that confer Timp inducibility. Fraction of adherent cells is shown for each of the genotypes. (Column 1) Adherent fractions were normalized to the number of adherent cells in uninduced cells transformed with wild-type NijA. Error bars indicate SEM. Data from three experiments are shown in columns 2 and 3; six experiments are shown in columns 4 and 5. (B) Wild-type S2 cells were treated with dsRNA against NijA to knock down the endogenous protein levels or mock treated with no RNA. Column 2 shows the fraction of adherent cells after dsRNA treatment, normalized to the number of adherent cells in column 1 (mock-treated). Error bars indicate SEM; data from three experiments are shown.
Figure 10.
Proposed mechanism of Mmp1 and NijA promoting the loss of cell adhesion. Our data indicate that Mmp1, shown as scissors, is responsible for shedding the NijA extracellular domain. The white cell expresses NijA and the gray cell does not. After cleavage, the liberated ectodomain of NijA binds to an unknown receptor (shown as a V) on the gray cell in a nonautonomous fashion. The gray cell then loses cell adhesion and releases from the substrate.
Similar articles
- Drosophila matrix metalloproteinases are required for tissue remodeling, but not embryonic development.
Page-McCaw A, Serano J, Santé JM, Rubin GM. Page-McCaw A, et al. Dev Cell. 2003 Jan;4(1):95-106. doi: 10.1016/s1534-5807(02)00400-8. Dev Cell. 2003. PMID: 12530966 - Dm1-MMP, a matrix metalloproteinase from Drosophila with a potential role in extracellular matrix remodeling during neural development.
Llano E, Pendás AM, Aza-Blanc P, Kornberg TB, López-Otín C. Llano E, et al. J Biol Chem. 2000 Nov 17;275(46):35978-85. doi: 10.1074/jbc.M006045200. J Biol Chem. 2000. PMID: 10964925 Retracted. - Matrix metalloproteinases promote motor axon fasciculation in the Drosophila embryo.
Miller CM, Page-McCaw A, Broihier HT. Miller CM, et al. Development. 2008 Jan;135(1):95-109. doi: 10.1242/dev.011072. Epub 2007 Nov 28. Development. 2008. PMID: 18045838 - Role of matrix metalloproteinases in melanoma cell invasion.
Hofmann UB, Houben R, Bröcker EB, Becker JC. Hofmann UB, et al. Biochimie. 2005 Mar-Apr;87(3-4):307-14. doi: 10.1016/j.biochi.2005.01.013. Biochimie. 2005. PMID: 15781317 Review. - Crystal structures of MMPs in complex with physiological and pharmacological inhibitors.
Maskos K. Maskos K. Biochimie. 2005 Mar-Apr;87(3-4):249-63. doi: 10.1016/j.biochi.2004.11.019. Biochimie. 2005. PMID: 15781312 Review.
Cited by
- A secreted MMP is required for reepithelialization during wound healing.
Stevens LJ, Page-McCaw A. Stevens LJ, et al. Mol Biol Cell. 2012 Mar;23(6):1068-79. doi: 10.1091/mbc.E11-09-0745. Epub 2012 Jan 19. Mol Biol Cell. 2012. PMID: 22262460 Free PMC article. - Noncanonical Decapentaplegic Signaling Activates Matrix Metalloproteinase 1 To Restrict Hedgehog Activity and Limit Ectopic Eye Differentiation in Drosophila.
Aggarwal P, Gera J, Ghosh S, Mandal L, Mandal S. Aggarwal P, et al. Genetics. 2017 Sep;207(1):197-213. doi: 10.1534/genetics.117.201053. Epub 2017 Jul 10. Genetics. 2017. PMID: 28696218 Free PMC article. - Relationship between nerve injury-induced protein gene 2 polymorphism and stroke in Chinese Han population.
Wang X, Zhang J, Liu Y, Zhang Y. Wang X, et al. J Biomed Res. 2011 Jul;25(4):287-91. doi: 10.1016/S1674-8301(11)60039-0. J Biomed Res. 2011. PMID: 23554703 Free PMC article. - Drosophila MMP2 regulates the matrix molecule faulty attraction (Frac) to promote motor axon targeting in Drosophila.
Miller CM, Liu N, Page-McCaw A, Broihier HT. Miller CM, et al. J Neurosci. 2011 Apr 6;31(14):5335-47. doi: 10.1523/JNEUROSCI.4811-10.2011. J Neurosci. 2011. PMID: 21471368 Free PMC article. - Remodeling the model organism: matrix metalloproteinase functions in invertebrates.
Page-McCaw A. Page-McCaw A. Semin Cell Dev Biol. 2008 Feb;19(1):14-23. doi: 10.1016/j.semcdb.2007.06.004. Epub 2007 Jul 6. Semin Cell Dev Biol. 2008. PMID: 17702617 Free PMC article. Review.
References
- Araki T., Milbrandt J. Ninjurin, a novel adhesion molecule, is induced by nerve injury and promotes axonal growth. Neuron. 1996;17:353–361. - PubMed
- Araki T., Zimonjic D.B., Popescu N.C., Milbrandt J. Mechanism of homophilic binding mediated by ninjurin, a novel widely expressed adhesion molecule. J. Biol. Chem. 1997;272:21373–21380. - PubMed
- Beitel G.J., Krasnow M.A. Genetic control of epithelial tube size in the Drosophila tracheal system. Development. 2000;127:3271–3282. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous