Clearance and prevention of prion infection in cell culture by anti-PrP antibodies - PubMed (original) (raw)
Clearance and prevention of prion infection in cell culture by anti-PrP antibodies
Joanna Pankiewicz et al. Eur J Neurosci. 2006 May.
Abstract
Prion diseases are transmissible and invariably fatal neurodegenerative disorders associated with a conformational transformation of the cellular prion protein (PrP(C)) into a self-replicating and proteinase K (PK)-resistant conformer, scrapie PrP (PrP(Sc)). Humoral immunity may significantly prolong the incubation period and even prevent disease in murine models of prionoses. However, the mechanism(s) of action of anti-PrP monoclonal antibodies (Mabs) remain(s) obscure. The murine neuroblastoma N2a cell line, infected with the 22L mouse-adapted scrapie strain, was used to screen a large library of Mabs with similar binding affinities to PrP, to identify those antibodies which could clear established infection and/or prevent infection de novo. Three Mabs were found capable of complete and persistent clearing of already-infected N2a cells of PrP(Sc). These antibodies were 6D11 (generated to PK-resistant PrP(Sc) and detecting PrP residues 93-109), and 7H6 and 7A12, which were raised against recombinant PrP and react with neighbouring epitopes of PrP residues 130-140 and 143-155, respectively. Mabs were found to interact with PrP(Sc) formation both on the cell surface and after internalization in the cytosol. Treatment with Mabs was not associated with toxicity nor did it result in decreased expression of PrP(C). Both preincubation of N2a cells with Mabs prior to exposure to 22L inoculum and preincubation of the inoculum with Mabs prior to infecting N2a cells resulted in a significant reduction in PrP(Sc) levels. Information provided in these studies is important for the rational design of humoral immune therapy for prion infection in animals and eventually in humans.
Figures
Fig. 1
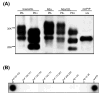
(A) Mab 6D11 detected di-, mono- and nonglycosylated PrP isoforms prior to and after PK treatment in brain homogenate from 22L-infected CD-1 mouse (brain/22L) and in cell lysate from N2a cells infected with the 22L prion strain (N2a/22L). 6D11 also reacted with PrPC produced by noninfected N2a cells (N2a) and with recombinant PrP (recPrP). Ten micrograms of total protein from brain homogenate and 40 and 10 μg of total protein from cell lysates were loaded onto lanes 1, 3 and 4, respectively. Two hundred micrograms of total protein from brain homogenate or cell lysate were subjected to PK digestion and loaded onto lanes 2 and 5, and 3 μg of recPrP was loaded onto lane 6. PK-, protein not treated with PK; PK+, protein treated with PK. (B) Dot–blot analysis of 6D11 binding epitope. 6D11 reacted with a peptide homologous to PrP residues 93–122 with an affinity similar to that shown for full-length recPrP.
Fig. 2
(A) PK-treated cell lysates from N2a murine neuroblastoma cells exposed to homogenate from a control brain (brain) or from a brain of a CD-1 mouse infected with 22L mouse-adapted scrapie strain (brain/22L). No PK-resistant material could be detected in cells cultured in the presence of control brain homogenate. Exposure of N2a cells to 22L mouse-adapted scrapie strain resulted in persistent infection of this line as the stable presence of PK-resistant PrPSc could be demonstrated in subsequent passages. (B) PK-treated cell lysates from N2a cells exposed to different mouse-adapted scrapie strains: 22L, 87V, 139A and ME7. Only exposure to the 22L strain resulted in the stable infection and sustained formation of PK-resistant PrPSc derived from PrPC expressed by the N2a cells. A weak signal can be seen in the first passage; this almost completely fades out in the second passage and is derived either from PrPSc carried over from the inoculum or from limited, unsustained conversion of cellular PrPC to PrPSc. No PrPSc could be detected in the third or higher passages of N2a cells exposed to 87V, 139A or ME7.
Fig. 3
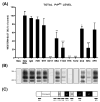
(A) The level of PrPSc in N2a/22L cells grown in the presence of anti-PrP Mabs. Mabs 6D11, 7H6 and 7A12 caused complete abrogation of PrPSc in infected cells. N2a (noninfected N2a cells), N2a/22L (N2a cells infected with the 22L strain) and IgG (N2a/22L cells incubated in the presence of murine IgG) were included as controls. OD, optical density; values are given as a mean + SD from at least three independent experiments. One-way ANOVA (P < 0.0001) was followed by Dunnett’s post hoc test; *P < 0.05, **P < 0.01, ***P < 0.001. (B) PK-resistant PrPSc in cell lysates following treatment with particular Mabs. (C) Schematic representation of epitopes of Mabs used to treat N2a/22L cells.
Fig. 4
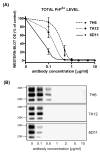
Dose-dependant inhibition of PrPSc formation in N2a/22L cells by Mabs. (A) Densitometric measurements of PrPSc bands detected in the Western blots fitted to sigmoidal dose–response curves. Values are given as mean ± SD from at least three independent experiments. (B) Western blots of PK-treated cell lysates from N2a/22L cells treated for 4 days with different concentrations of Mabs.
Fig. 5
(A) Absence of PK-resistant PrPSc in N2a/22L cells incubated with Mabs 6D11, 7H6 and 7A12 for 8 days. (B) There was no reappearance of PrPSc after the cells were grown for another 14 days in the absence of Mabs. This indicates that the effect of treatment was persistent.
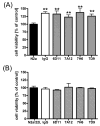
Fig. 6
Protein expression in N2a/22L cells treated with Mabs 2C2, 6D11 and 7H6. (A) Level of PrPSc following PK digestion; (B) level of total PrP; (C) level of β-actin.
Fig. 7
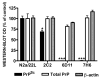
Quantification of protein expression in N2a/22L cells treated with Mabs. While 2C2 resulted in partial reduction in PrPSc and 6D11 and 7H6 caused complete abrogation of PrPSc, treatment with these Mabs did not significantly affect either the total level of PrP (PrPC + PrPSc) or the level of unrelated proteins such as β-actin. Values are given as mean + SD from at least three independent experiments. One-way ANOVA (P < 0.0001) was followed by Dunnett’s post hoc test; *P < 0.05, ***P < 0.001.
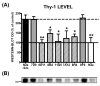
Fig. 8
(A) The level of Thy-1 in N2a/22L cells treated with various Mabs. The level of Thy-1 in N2a/22L cells was more than twice than in N2a cells. Mabs 7D9 and 8F9, which showed no significant therapeutic effect, had no impact on the Thy-1 level. Incubation of N2a/22L cells with therapeutically effective Mabs caused a significant reduction in Thy-1 level. One-way ANOVA (P < 0.001) was followed by a Dunnett post hoc test; *P < 0.05, **P < 0.01. (B) Thy-1 in cell lysates following treatment with specific Mabs.
Fig. 9
The effect of Mabs on viability of N2a and N2a/22L cells. (A) Murine IgG and anti-PrP Mabs had a nonspecific trophic effect on N2a cells. One-way ANOVA (P < 0.0001) was followed by Dunnett’s post hoc test; **P < 0.01. (B) Anti-PrP Mabs showed neither a trophic effect nor reduced viability of N2a/22L cells. Values are given as a mean + SD from six independent measurements.
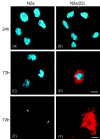
Fig. 10
Incubation of N2a and N2a/22L cells in the presence of Mab 6D11 conjugated with Cy3 (6D11/Cy3). Labeling of the plasma membrane in N2a cells grown in the presence of 6D11/Cy3 for (A) 24 h and (C) 72 h. (E) Confocal microscopy images showing binding of Mabs to the outer leaflet of the plasma membrane (arrowheads). (B) In addition to membrane labeling, N2a/22L cells showed internalization of 6D11/Cy3 after 24 h, which (D and F) was more pronounced after 72 h. A–D, deconvolution microscope; E and F, confocal microscope. Red staining in A–F represents Cy3; blue staining in A–D is DAPI nuclear dye. Scale bars, 10 μm (in D for A–D), 20 μm (in F for E and F).
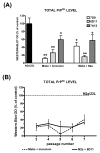
Fig. 11
(A) Decreased level of PrPSc in the third passage of N2a/22L cells which were infected with inoculum preincubated with Mabs (Mabs + inoculum) or N2a cells incubated with Mabs before being exposed to the inoculum (Mabs + N2a); results are compared to the level of PrPSc in N2a/22L cells infected in a standard manner. Values are given as mean + SD from at least three independent experiments. One-way ANOVA (P < 0.0001) was followed by Dunnett’s post hoc test; *P < 0.05, **P < 0.01. (B) Persistently decreased level of PrPSc was maintained in subsequent passages of N2a cells infected in the presence of 6D11. Values are given as mean ± SD from at least three independent experiments. Comparison was made to the density of PrPSc bands (designated as 100%) in corresponding passages of N2a/22L cells infected in the standard manner (repeated-measures ANOVA; P < 0.001).
Fig. 12
Kaplan–Meier survival analysis of CD-1 mice prophylactically treated with a single dose of Mab 7D9 administered immediately after intraperitoneal exposure to 22L brain homogenate. Animals which received Mab 7D9 showed a statically significant delay in onset of neurological symptoms compared to those which received an equivalent dose of IgG. Log rank test indicates a statistically significant difference between the groups (P < 0.0001).
Similar articles
- Anti-prion Protein Antibody 6D11 Restores Cellular Proteostasis of Prion Protein Through Disrupting Recycling Propagation of PrPSc and Targeting PrPSc for Lysosomal Degradation.
Pankiewicz JE, Sanchez S, Kirshenbaum K, Kascsak RB, Kascsak RJ, Sadowski MJ. Pankiewicz JE, et al. Mol Neurobiol. 2019 Mar;56(3):2073-2091. doi: 10.1007/s12035-018-1208-4. Epub 2018 Jul 9. Mol Neurobiol. 2019. PMID: 29987703 Free PMC article. - Anti-PrP antibodies block PrPSc replication in prion-infected cell cultures by accelerating PrPC degradation.
Perrier V, Solassol J, Crozet C, Frobert Y, Mourton-Gilles C, Grassi J, Lehmann S. Perrier V, et al. J Neurochem. 2004 Apr;89(2):454-63. doi: 10.1111/j.1471-4159.2004.02356.x. J Neurochem. 2004. PMID: 15056288 Free PMC article. - Vaccination with prion peptide-displaying papillomavirus-like particles induces autoantibodies to normal prion protein that interfere with pathologic prion protein production in infected cells.
Handisurya A, Gilch S, Winter D, Shafti-Keramat S, Maurer D, Schätzl HM, Kirnbauer R. Handisurya A, et al. FEBS J. 2007 Apr;274(7):1747-58. doi: 10.1111/j.1742-4658.2007.05721.x. Epub 2007 Feb 20. FEBS J. 2007. PMID: 17313482 Free PMC article. - Vaccine approaches to prevent and treat prion infection : progress and challenges.
Müller-Schiffmann A, Korth C. Müller-Schiffmann A, et al. BioDrugs. 2008;22(1):45-52. doi: 10.2165/00063030-200822010-00005. BioDrugs. 2008. PMID: 18215090 Review. - Prion protein-specific antibodies for therapeutic intervention of transmissible spongiform encephalopathies.
Buchholz CJ, Bach P, Nikles D, Kalinke U. Buchholz CJ, et al. Expert Opin Biol Ther. 2006 Mar;6(3):293-300. doi: 10.1517/14712598.6.3.293. Expert Opin Biol Ther. 2006. PMID: 16503737 Review.
Cited by
- Prion protein E219K polymorphism: from the discovery of the KANNO blood group to interventions for human prion disease.
Wang SS, Meng ZL, Zhang YW, Yan YS, Li LB. Wang SS, et al. Front Neurol. 2024 Jul 10;15:1392984. doi: 10.3389/fneur.2024.1392984. eCollection 2024. Front Neurol. 2024. PMID: 39050130 Free PMC article. Review. - Protective anti-prion antibodies in human immunoglobulin repertoires.
Senatore A, Frontzek K, Emmenegger M, Chincisan A, Losa M, Reimann R, Horny G, Guo J, Fels S, Sorce S, Zhu C, George N, Ewert S, Pietzonka T, Hornemann S, Aguzzi A. Senatore A, et al. EMBO Mol Med. 2020 Sep 7;12(9):e12739. doi: 10.15252/emmm.202012739. Epub 2020 Aug 10. EMBO Mol Med. 2020. PMID: 32776637 Free PMC article. - Unique structural properties associated with mouse prion Δ105-125 protein.
Patel A, Vasiljevic S, Jones IM. Patel A, et al. Prion. 2013 May-Jun;7(3):235-43. doi: 10.4161/pri.24429. Prion. 2013. PMID: 23764837 Free PMC article. - GFP-tagged mutant prion protein forms intra-axonal aggregates in transgenic mice.
Medrano AZ, Barmada SJ, Biasini E, Harris DA. Medrano AZ, et al. Neurobiol Dis. 2008 Jul;31(1):20-32. doi: 10.1016/j.nbd.2008.03.006. Epub 2008 Apr 7. Neurobiol Dis. 2008. PMID: 18514536 Free PMC article. - Prion protein expression and functional importance in skeletal muscle.
Smith JD, Moylan JS, Hardin BJ, Chambers MA, Estus S, Telling GC, Reid MB. Smith JD, et al. Antioxid Redox Signal. 2011 Nov 1;15(9):2465-75. doi: 10.1089/ars.2011.3945. Epub 2011 Jun 8. Antioxid Redox Signal. 2011. PMID: 21453198 Free PMC article.
References
- Adler V, Zeller B, Kryukov V, Kascsak R, Rubenstein R, Grossman A. Small, highly structured RNAs participate in the conversion of human recombinant Prp (Sen) to Prp (Res) in vitro . J Mol Biol. 2003;332:47–57. - PubMed
- Balter M. Spongiform disease. Experts downplay new vCJD fears. Science. 2002;289:1866–1867. - PubMed
- Carp RI, Meeker HC, Rubenstein R, Sigurdarson S, Papini M, Kascsak RJ, Kozlowski PB, Wisniewski HM. Characteristics of scrapie isolates derived from hay mites. J Neurovirol. 2000;6:137–144. - PubMed
- DeArmond SJ, Kretzschmar H, Prusiner SB. Prion diseases. In: Graham DI, Lantos P, editors. Greenfield’s Neuropathology. Arnold; London: 2002. pp. 273–324.
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS047433/NS/NINDS NIH HHS/United States
- AG15408/AG/NIA NIH HHS/United States
- N01-NS-02327/NS/NINDS NIH HHS/United States
- R01 NS045981/NS/NINDS NIH HHS/United States
- NS47433/NS/NINDS NIH HHS/United States
- R03 TW006848/TW/FIC NIH HHS/United States
- R01 AG015408/AG/NIA NIH HHS/United States
- NS45981/NS/NINDS NIH HHS/United States
- AG24847/AG/NIA NIH HHS/United States
- K08 AG024847/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials