A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis - PubMed (original) (raw)
Comparative Study
. 2006 Jul 10;203(7):1685-91.
doi: 10.1084/jem.20060285. Epub 2006 Jul 3.
Affiliations
- PMID: 16818675
- PMCID: PMC2118338
- DOI: 10.1084/jem.20060285
Comparative Study
A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis
Caroline Sutton et al. J Exp Med. 2006.
Abstract
It was recently demonstrated that interleukin (IL)-23-driven IL-17-producing (ThIL-17) T cells mediate inflammatory pathology in certain autoimmune diseases. We show that the induction of antigen-specific ThIL-17 cells, but not T helper (Th)1 or Th2 cells, by immunization with antigens and adjuvants is abrogated in IL-1 receptor type I-deficient (IL-1RI(-/-)) mice. Furthermore, the incidence of experimental autoimmune encephalomyelitis (EAE) was significantly lower in IL-1RI(-/-) compared with wild-type mice, and this correlated with a failure to induce autoantigen-specific ThIL-17 cells, whereas induction of Th1 and Th2 responses was not substantially different. However, EAE was induced in IL-1RI(-/-) mice by adoptive transfer of autoantigen-specific cells from wild-type mice with EAE. IL-23 alone did not induce IL-17 production by T cells from IL-1RI(-/-) mice, and IL-23-induced IL-17 production was substantially enhanced by IL-1alpha or IL-1beta, even in the absence of T cell receptor stimulation. We demonstrate essential roles for phosphatidylinositol 3-kinase, nuclear factor kappaB, and novel protein kinase C isoforms in IL-1- and IL-23-mediated IL-17 production. Tumor necrosis factor alpha also synergized with IL-23 to enhance IL-17 production, and this was IL-1 dependent. Our findings demonstrate that IL-1 functions upstream of IL-17 to promote pathogenic ThIL-17 cells in EAE.
Figures
Figure 1.
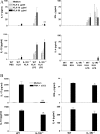
IL-1RI is required for induction or activation of antigen-specific ThIL-17 T cells. (A) C57BL/6 or IL-1RI−/− mice were immunized s.c. with 10 μg KLH either alone or with 10 μg LPS. Inguinal lymph nodes were harvested after 7 d and cells were restimulated with medium or 2, 10, or 50 μg/ml KLH. (B) Lymph node cells were restimulated with medium or 20 ng/ml PMA and 1 μg/ml anti-CD3. Supernatants were collected after 3 d, and IL-4, IL-10, IL-17, and IFN-γ concentrations were determined by ELISA. C57BL/6 versus IL-1RI−/−: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Figure 2.
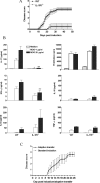
The induction of EAE and autoantigen-specific ThIL-17 cells is dependent on functional IL-1RI. C57BL/6 or IL-1RI−/− mice were injected with 150 μg MOG in 200 μl CFA and 500 ng PT followed 2 d later with a second dose of PT. Mice were observed for clinical symptoms of EAE daily for 48 d. A separate group of mice were killed on d 21, and spleen cells were restimulated with MOG peptide. After 3 d, supernatants were collected and cytokine concentrations were determined by ELISA. Cell proliferation was determined on day 4 of culture by [3H]thymidine incorporation. (A) Disease scores (n = 15) ± SD. (B) Antigen-specific cytokine production and proliferation. C57BL/6 versus IL-1RI−/−: *, P < 0.05; **, P < 0.01; ***, P < 0.001. (C) IL-1RI−/− mice (n = 7) were injected with MOG in CFA followed by PT as described above or with MOG-specific T cells (9 × 106 cells/mouse) generated from the spleens of C57BL/6 mice with EAE. Disease scores (n = 5) ± SD.
Figure 3.
IL-1 synergizes with IL-23 to promote IL-17 production by spleen cells and purified CD4+ and CD8+ T cells. (A) Spleen cells (106 cells/ml) from C57BL/6 or IL-1RI−/− mice were cultured with 100 pg/ml IL-23 alone or together with 1 ng/ml IL-1α, and supernatants were collected after 3 d for analysis of IL-17 and IFN-γ by ELISA. C57BL/6 versus IL-1RI−/−: *, P < 0.05; **, P < 0.01; ***, P < 0.001. IL-1α plus IL-23 versus IL-23; #, P < 0.001. (B) Spleen cells (2.25 × 106 cells/ml) from C57BL/6 mice were cultured for 48 h with medium or cytokines and for the last 4 h in the presence of 10 μg/ml brefeldin A. Intracellular staining for IL-17 and IFN-γ was performed. Percentages refer to IL-17 production by gated CD3+ T cells. Splenic CD4+ (C) and CD8+ (D) T cells (5 × 105cells/ml) from C57BL/6 mice were incubated with 10 U/ml IL-2 and 1 μg/ml anti-CD3 antibody alone or together with 0.1–1 ng/ml IL-23 and/or 1 ng/ml IL-1α or IL-1β. IL-17 concentrations were measured by ELISA in supernatants collected after 3 d. IL-23 plus IL-1 versus IL-23 only: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Figure 4.
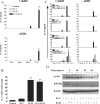
IL-1 and IL-23 promote IL-17 production in the absence of TCR stimulation via stimulation of PI3K, NF-κB, and PKCθ. (A) Splenic CD3+ cells (5 × 105 cells/ml) from C57BL/6 mice were incubated with 10 ng/ml IL-1β, 10 ng/ml IL-23, IL-1 and IL-23, or medium only in the presence or absence of anti-CD3, and supernatants were collected at 12, 24, 48, or 72 h for analysis by ELISA. (B) Splenic CD3+ cells from C57BL/6 mice were incubated with 1 μg/ml anti-CD3 for 2 h before stimulation with IL-1, IL-23, IL-1 and IL-23, or medium. IL-17 mRNA expression was evaluated by real-time PCR 48 h after stimulation, and the data represent the fold change for IL-17 mRNA in relation to 18S rRNA. IL-23 plus IL-1 versus IL-23 only: *, P < 0.05; **, P < 0.01; ***, P < 0.001. (C) CD3+ cells (5 × 105 cells/ml) were stimulated with medium (control) or with IL-23, IL-1, or IL-23 and IL-1 with or without anti-CD3 in the presence or absence of (30-min pretreatment) LY294002 (PI3K inhibitor at 20 and 40 μM), BAY 11-7082 (NF-κB inhibitor at 4 and 8 μM), rottlerin (novel PKC inhibitor at 10 and 100 μM), UO126 (ERK inhibitor at 5 μM), or SB203580 (p38 inhibitor at 5 μM). Supernatants were collected 3 d later for analysis of IL-17 production by ELISA. IL-23 plus IL-1 versus IL-23 plus IL-1 plus inhibitor: *, P < 0.05; **, P < 0.01; ***, P < 0.001. (D) CD3+ cells were incubated with medium only or with IL-1, IL-23, or IL-1 and IL-23. Cell lysates were analyzed by Western blotting with antibodies specific for phosphorylated Akt and IκB-α or β-actin as a loading control.
Figure 5.
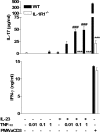
TNF-α promotes IL-23–induced IL-17 production in an IL-1–dependent manner. Spleen cells (106 cells/ml) from C57BL/6 or IL-1RI−/− were cultured with 1 ng/ml IL-23, 0.01–1 ng/ml TNF-α, 0.01–1 ng/ml IL-23 and TNF-α, medium, or PMA and anti-CD3. Supernatants were collected after 3 d for analysis of IL-17 and IFN-γ production by ELISA. C57BL/6 versus IL-1RI−/−: *, P < 0.05; **, P < 0.01; ***, P < 0.001. TNF-α plus IL-23 versus IL-23: #, P < 0.05; ##, P < 0.01; ###, P < 0.001.
Similar articles
- Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity.
Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Sutton CE, et al. Immunity. 2009 Aug 21;31(2):331-41. doi: 10.1016/j.immuni.2009.08.001. Epub 2009 Aug 13. Immunity. 2009. PMID: 19682929 - CCR7 ligands are required for development of experimental autoimmune encephalomyelitis through generating IL-23-dependent Th17 cells.
Kuwabara T, Ishikawa F, Yasuda T, Aritomi K, Nakano H, Tanaka Y, Okada Y, Lipp M, Kakiuchi T. Kuwabara T, et al. J Immunol. 2009 Aug 15;183(4):2513-21. doi: 10.4049/jimmunol.0800729. Epub 2009 Jul 22. J Immunol. 2009. PMID: 19625643 - CD4+ICOS+ T lymphocytes inhibit T cell activation 'in vitro' and attenuate autoimmune encephalitis 'in vivo'.
Rojo JM, Pini E, Ojeda G, Bello R, Dong C, Flavell RA, Dianzani U, Portolés P. Rojo JM, et al. Int Immunol. 2008 Apr;20(4):577-89. doi: 10.1093/intimm/dxn016. Epub 2008 Feb 28. Int Immunol. 2008. PMID: 18310064 - Pathophysiology of interleukin-23 in experimental autoimmune encephalomyelitis.
Touil T, Fitzgerald D, Zhang GX, Rostami AM, Gran B. Touil T, et al. Drug News Perspect. 2006 Mar;19(2):77-83. doi: 10.1358/dnp.2006.19.2.977443. Drug News Perspect. 2006. PMID: 16628262 Review. - Caspase-1-processed IL-1 family cytokines play a vital role in driving innate IL-17.
Dungan LS, Mills KH. Dungan LS, et al. Cytokine. 2011 Oct;56(1):126-32. doi: 10.1016/j.cyto.2011.07.007. Epub 2011 Aug 6. Cytokine. 2011. PMID: 21824786 Review.
Cited by
- Cord factor and peptidoglycan recapitulate the Th17-promoting adjuvant activity of mycobacteria through mincle/CARD9 signaling and the inflammasome.
Shenderov K, Barber DL, Mayer-Barber KD, Gurcha SS, Jankovic D, Feng CG, Oland S, Hieny S, Caspar P, Yamasaki S, Lin X, Ting JP, Trinchieri G, Besra GS, Cerundolo V, Sher A. Shenderov K, et al. J Immunol. 2013 Jun 1;190(11):5722-30. doi: 10.4049/jimmunol.1203343. Epub 2013 Apr 29. J Immunol. 2013. PMID: 23630357 Free PMC article. - Role of murine intestinal interleukin-1 receptor 1-expressing lymphoid tissue inducer-like cells in Salmonella infection.
Chen VL, Surana NK, Duan J, Kasper DL. Chen VL, et al. PLoS One. 2013 Jun 4;8(6):e65405. doi: 10.1371/journal.pone.0065405. Print 2013. PLoS One. 2013. PMID: 23750260 Free PMC article. - IL-17 mediates immunopathology in the absence of IL-10 following Leishmania major infection.
Gonzalez-Lombana C, Gimblet C, Bacellar O, Oliveira WW, Passos S, Carvalho LP, Goldschmidt M, Carvalho EM, Scott P. Gonzalez-Lombana C, et al. PLoS Pathog. 2013 Mar;9(3):e1003243. doi: 10.1371/journal.ppat.1003243. Epub 2013 Mar 21. PLoS Pathog. 2013. PMID: 23555256 Free PMC article. - Interleukin-1 promotes autoimmune neuroinflammation by suppressing endothelial heme oxygenase-1 at the blood-brain barrier.
Hauptmann J, Johann L, Marini F, Kitic M, Colombo E, Mufazalov IA, Krueger M, Karram K, Moos S, Wanke F, Kurschus FC, Klein M, Cardoso S, Strauß J, Bolisetty S, Lühder F, Schwaninger M, Binder H, Bechman I, Bopp T, Agarwal A, Soares MP, Regen T, Waisman A. Hauptmann J, et al. Acta Neuropathol. 2020 Oct;140(4):549-567. doi: 10.1007/s00401-020-02187-x. Epub 2020 Jul 11. Acta Neuropathol. 2020. PMID: 32651669 Free PMC article. - Endogenous interleukin-10 constrains Th17 cells in patients with inflammatory bowel disease.
Wilke CM, Wang L, Wei S, Kryczek I, Huang E, Kao J, Lin Y, Fang J, Zou W. Wilke CM, et al. J Transl Med. 2011 Dec 16;9:217. doi: 10.1186/1479-5876-9-217. J Transl Med. 2011. PMID: 22176654 Free PMC article.
References
- Steinman, L. 2001. Multiple sclerosis: a two-stage disease. Nat. Immunol. 2:762–764. - PubMed
- McKenzie, B.S., R.A. Kastelein, and D.J. Cua. 2006. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 27:17–23. - PubMed
- Krakowski, M., and T. Owens. 1996. Interferon-gamma confers resistance to experimental allergic encephalomyelitis. Eur. J. Immunol. 26:1641–1646. - PubMed
- Cua, D.J., J. Sherlock, Y. Chen, C.A. Murphy, B. Joyce, B. Seymour, L. Lucian, W. To, S. Kwan, T. Churakova, et al. 2003. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 421:744–748. - PubMed
- Aggarwal, S., N. Ghilardi, M.H. Xie, F.J. de Sauvage, and A.L. Gurney. 2003. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 278:1910–1914. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases