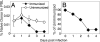Cutting edge: latecomer CD8 T cells are imprinted with a unique differentiation program - PubMed (original) (raw)
Cutting edge: latecomer CD8 T cells are imprinted with a unique differentiation program
Warren N D'Souza et al. J Immunol. 2006.
Abstract
Factors that influence T cell responses, such as Ag load, APCs, costimulatory molecules, and cytokines, dramatically change during the course of an immune response. We observed that antiviral CD8 T cells were not recruited from circulation simultaneously, but over a period of 3-4 days. Consequently, locally resident T cells and those that entered secondary lymphoid tissue later were primed in very different environments. The cells recruited later in the response were imprinted with a unique differentiation program, such that their magnitude of proliferation was reduced and their kinetics of expansion was delayed. In addition, we found that the "latecomer" CD8 T cells displayed a unique surface phenotype indicative of reduced stimulation but were not preferentially recruited into the surviving pool of memory cells. This finding demonstrates that the timing of recruitment of individual T cell clones determines the population dynamics of the subsequent immune response.
Figures
FIGURE 1
Antiviral CD8 T cells are not recruited simultaneously. CFSE-labeled OT-I/RAG−/−/CD45.1+ cells (1 × 106) were transferred to B6 (CD45.2+) hosts that were left unimmunized or were immunized with VSVOVA a day later. A, Percentage of naive donor cells in PBL was measured over time. B, Donor cell numbers present within immunized mice were expressed as a percentage of those in unimmunized animals. The values plotted represent the mean ± SD.
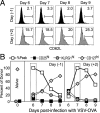
FIGURE 2
Latecomer CD8 T cells exhibit a diminished and delayed peak of expansion. Four groups of B6 (CD45.2+) mice were immunized with VSV-OVA on day 0. One group of mice did not receive any OT-I cells, whereas the other three groups received OT-I cells on day −1, day +2, or day +4, respectively. The endogenous response to OVA (Endog) in the mice that did not receive any OT-I cells (None) or in those that received OT-I cells at indicated time points (d(−1), d(+ 2), and d(+4), representing days −1, +2, and +4, respectively) was tracked using MHC class I tetramers (Kb-SIINFEKL), whereas the presence of the donor OT-I cells was determined using the congenic marker CD45.1. A_−_C, Mice were analyzed at indicated time points following infection, and numbers were plotted as a percentage of total PBL. The response within the mice that did not receive any OT-I cells (None Endog) is plotted again in B and C for reference. D and E, The expansion and contraction of each subpopulation was normalized to the peak of expansion.
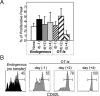
FIGURE 3
Latecomer cells display a phenotype indicative of reduced stimulation. Experiments were performed as in Fig. 2, where mice received day −1 or day +2 OT-I cells and were immunized with VSV-OVA at day 0. The expression of CD62L (L-selectin) (A) and that of CD25, KLRG1, and CD127 (IL-7Rα)(B) on the OT-I cells in the spleen were determined at days 6, 7, 8, and 9 postinfection. In B, the normalized response kinetics taken from Fig. 2_E_ (percent peak) are plotted for reference along with the percentage of donor cells expressing the indicated markers. Also indicated (left panel) is the expression of the cell surface markers on naive OT-I cells before transfer.
FIGURE 4
Similar proportions of latecomer T cells survive the contraction phase. Experiments were performed as in Fig. 2. The endogenous and the OT-I responses in PBL were tracked during all phases of the response. A, The efficiency of memory generation was calculated by expressing the proportion of memory cells present in PBL (at day 71) as a percentage of the respective proliferative peaks. B, The expression of CD62L on the indicated cell populations in the spleen was determined at ~3 mo postinfection. Numbers plotted are the percentage of cells that are CD62Lhigh.
FIGURE 5
Latecomer T cells are capable of differentiating into functional memory cells. Experiments were performed as in Fig. 2. At ~4 mo postinfection, the percentage of endogenous SIINFEKL-specific cells (tetramer+CD45.1−) and donor OT-I cells (tetramer+CD45.1+) in PBL was determined. The mice were then challenged with Lm-OVA a day later and analyzed at day 7 postchallenge. Cells were gated on CD8+ cells, and the numbers indicated are the percentages of total PBL.
Similar articles
- Reducing the stimulation of CD8+ T cells during infection with intracellular bacteria promotes differentiation primarily into a central (CD62LhighCD44high) subset.
van Faassen H, Saldanha M, Gilbertson D, Dudani R, Krishnan L, Sad S. van Faassen H, et al. J Immunol. 2005 May 1;174(9):5341-50. doi: 10.4049/jimmunol.174.9.5341. J Immunol. 2005. PMID: 15843531 - CD40 ligation in vivo induces bystander proliferation of memory phenotype CD8 T cells.
Koschella M, Voehringer D, Pircher H. Koschella M, et al. J Immunol. 2004 Apr 15;172(8):4804-11. doi: 10.4049/jimmunol.172.8.4804. J Immunol. 2004. PMID: 15067057 - The dual role of IL-2 in the generation and maintenance of CD8+ memory T cells.
Dai Z, Konieczny BT, Lakkis FG. Dai Z, et al. J Immunol. 2000 Sep 15;165(6):3031-6. doi: 10.4049/jimmunol.165.6.3031. J Immunol. 2000. PMID: 10975812 - Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells.
Schluns KS, Williams K, Ma A, Zheng XX, Lefrançois L. Schluns KS, et al. J Immunol. 2002 May 15;168(10):4827-31. doi: 10.4049/jimmunol.168.10.4827. J Immunol. 2002. PMID: 11994430
Cited by
- Optimising IL-2 for Cancer Immunotherapy.
Sprent J, Boyman O. Sprent J, et al. Immune Netw. 2024 Jan 26;24(1):e5. doi: 10.4110/in.2024.24.e5. eCollection 2024 Feb. Immune Netw. 2024. PMID: 38455463 Free PMC article. Review. - Tissue-specific abundance of interferon-gamma drives regulatory T cells to restrain DC1-mediated priming of cytotoxic T cells against lung cancer.
Zagorulya M, Yim L, Morgan DM, Edwards A, Torres-Mejia E, Momin N, McCreery CV, Zamora IL, Horton BL, Fox JG, Wittrup KD, Love JC, Spranger S. Zagorulya M, et al. Immunity. 2023 Feb 14;56(2):386-405.e10. doi: 10.1016/j.immuni.2023.01.010. Epub 2023 Feb 2. Immunity. 2023. PMID: 36736322 Free PMC article. - Regulation of effector and memory CD8 + T cell differentiation: a focus on orphan nuclear receptor NR4A family, transcription factor, and metabolism.
Oladipo OO, Adedeji BO, Adedokun SP, Gbadamosi JA, Salaudeen M. Oladipo OO, et al. Immunol Res. 2023 Jun;71(3):314-327. doi: 10.1007/s12026-022-09353-1. Epub 2022 Dec 26. Immunol Res. 2023. PMID: 36571657 Review. - Cytomegalovirus-specific T cells restricted for shared and donor human leukocyte antigens differentially impact on cytomegalovirus reactivation risk after allogeneic hematopoietic stem cell transplantation.
Tassi E, Noviello M, De Simone P, Lupo-Stanghellini MT, Doglio M, Serio F, Abbati D, Beretta V, Valtolina V, Oliveira G, Racca S, Campodonico E, Ruggiero E, Clerici D, Giglio F, Lorentino F, Dvir R, Xue E, Farina F, Oltolini C, Manfredi F, Vago L, Corti C, Bernardi M, Clementi M, Brix L, Ciceri F, Peccatori J, Greco R, Bonini C. Tassi E, et al. Haematologica. 2023 Jun 1;108(6):1530-1543. doi: 10.3324/haematol.2022.280685. Haematologica. 2023. PMID: 36200418 Free PMC article. - Aging-Related Cellular, Structural and Functional Changes in the Lymph Nodes: A Significant Component of Immunosenescence? An Overview.
Cakala-Jakimowicz M, Kolodziej-Wojnar P, Puzianowska-Kuznicka M. Cakala-Jakimowicz M, et al. Cells. 2021 Nov 12;10(11):3148. doi: 10.3390/cells10113148. Cells. 2021. PMID: 34831371 Free PMC article. Review.
References
- Wong P, Pamer EG. Cutting edge: antigen-independent CD8 T cell proliferation. J. Immunol. 2001;166:5864–5868. - PubMed
- van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat. Immunol. 2001;2:423–429. - PubMed
- Gett AV, Sallusto F, Lanzavecchia A, Geginat J. T cell fitness determined by signal strength. Nat. Immunol. 2003;4:355–360. - PubMed
- van Stipdonk MJ, Hardenberg G, Bijker MS, Lemmens EE, Droin NM, Green DR, Schoenberger SP. Dynamic programming of CD8+ T lymphocyte responses. Nat. Immunol. 2003;4:361–365. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 AI037988/AI/NIAID NIH HHS/United States
- R37 AI 21372/AI/NIAID NIH HHS/United States
- R37 AI021372-22/AI/NIAID NIH HHS/United States
- R37 AI021372/AI/NIAID NIH HHS/United States
- R01 AI 37988/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous