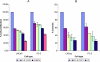Akt-and CREB-mediated prostate cancer cell proliferation inhibition by Nexrutine, a Phellodendron amurense extract - PubMed (original) (raw)
Akt-and CREB-mediated prostate cancer cell proliferation inhibition by Nexrutine, a Phellodendron amurense extract
Gretchen E Garcia et al. Neoplasia. 2006 Jun.
Abstract
Evidence from epidemiological studies suggests that plant-based diets can reduce the risk of prostate cancer. However, very little information is available concerning the use of botanicals in preventing prostate cancer. As a first step toward developing botanicals as prostate cancer preventives, we examined the effect of Nexrutine on human prostate cancer cells. Nexrutine is a herbal extract developed from Phellodendron amurense. Phellodendron extracts have been used traditionally in Chinese medicine for hundreds of years as an antidiarrheal, astringent, and anti-inflammatory agent. The present study investigated its potential antitumor effect on human prostate cancer cells. Our results suggest that it inhibits tumor cell proliferation through apoptosis induction and inhibition of cell survival signaling. The results of the present study indicate that Nexrutine treatment 1) inhibits the proliferation of both androgen-responsive and androgen-independent human prostate cancer cells through induction of apoptosis; 2) reduces levels of pAkt, phosphorylated cAMP response-binding protein (pCREB) and CREB DNA-binding activity; and 3) induces apoptosis in prostate cancer cells stably overexpressing Bcl-2. Further, Akt kinase activity was reduced in cells treated with Nexrutine, and ectopic expression of myristoylated Akt protected from Nexrutine induced inhibition of proliferation, implicating a role for Akt signaling.
Figures
Figure 1
Nexrutine inhibits anchorage-dependent (A) and anchorage-independent (B) growth of human PCA cells. Androgen-responsive (LNCaP) and androgenindependent (PC-3) cells were plated in 96-well plates as described in Materials and Methods and treated with indicated concentrations of either Nexrutine (in micrograms per milliliter) or solvent control. Cell proliferation was determined using Cell Titer96 Aqueous One solution assay at 72 hours. Absorbance at 570 nm determined by using a SpectraMaxPlus plate reader (Molecular Devices, Sunnyvale, CA) is shown. The data shown are averages ± S.D. of three replicate wells and is representative of four independent experiments. (B) Anchorage-independent growth cells were plated in triplicate in 35-mm dishes on 0.5% agarose containing medium as described in Materials and Methods. After 14 days' incubation, cells were stained with 0.5 ml of 0.02% p-iodonitrotetrazolium and colonies were counted in 10 different fields from each plate. The results are expressed as means ± S.D. and are representative of two independent experiments.
Figure 2
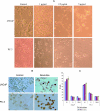
Nexrutine treatment-induced apoptosis. (A) Morphological alterations; (B) apoptosis determined by TUNEL staining. LNCaP and PC-3 cells were treated with either DMSO or Nexrutine (0, 1,2.5, and 5 µg/ml) for 24 hours. (A) Photomicrographs of cells by phase-contrast microscopy using a Nikon Microscope with a digital camera system Coolpix 995 (Nikon Corporation, Tokyo, Japan). Original magnification x 20. (B) TUNEL staining of LNCaP and PC-3 cells treated with 5 µg/ml Nexrutine for 24 hours. Brown-stained nuclei are indicative of apoptosis. (C) Effect of Nexrutine for 24 hours on cell cycle distribution. The data shown are averages ± S.D. of two independent experiments.
Figure 3
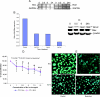
Bcl-2 may not be a direct target of Nexrutine-induced biologic effects. (A). Expression of Bcl-2 and Bax was examined using quantitative RT-PCR and Western blotting. Total RNA and whole-cell protein extracts were prepared from LNCaP cells treated with Nexrutine (5 µg/ml for different time intervals). RNA was used in quantitative RT-PCR with Bcl-2/GAPDH or Bax/GAPDH primers. A representative gel is shown. The bottom band in each lane is GAPDH. (B). Levels of Bcl-2, Bax, and GAPDH were quantified with KODAK MI software using KODAK Image Station 4000R digital imaging system (Eastman Kodak Company, Kodak Imaging Systems, New Haven, CT). The ratio of Bcl-2/Bax expression was determined after normalization with GAPDH internal control and shown as a graph. (C) Equal amounts of the extracts were fractionated on a 10% SDS-polyacrylamide gel and transferred onto a nitrocellulose membrane. The blotted membrane was blocked with 5% nonfat dry milk in TBS containing 0.1% Tween 20 (blocking solution), and incubated with indicated antibodies followed by incubation with HRP-conjugated anti-rabbit IgG antibody (Sigma) in blocking solution. Bound antibody was detected by enhanced chemiluminescence using Supersignal West Pico Chemiluminescent Substrate following the manufacturer's directions (Pierce, Rockford, IL). The blot shown is a representative blot of three different experiments. All the blots were stripped and reprobed with β-actin to ensure equal protein loading. (D) PC3-Neo (Neo) and PC-3 Bcl-2 (Bcl-2) cells were used in proliferation assay. Cells were plated in 96-well plates as described in Figure 1A and treated with indicated concentrations of either Nexrutine or solvent control. Cell proliferation was measured by Cell Titer96 Aqueous One solution assay at 72-hour time point by determining the absorbance at 570 nm using SpectraMaxPlus plate reader (Molecular Devices). Statistical significance of the data was determined by Student's t test. (E) PC-3 Neo and PC-3 Bcl-2 cells treated with Nexrutine (5 µg/ml for 24 hours) were trypsinized and used in DAPI staining as described in Materials and Methods. Bright fluorescence indicates viable cells.
Figure 4
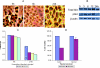
Nexrutine treatment reduces the expression of pAkt. (A) Cells treated with Nexrutine (5 µg/ml for 24 hours) were fixed on slides using paraformaldehyde. Fixed slides were blocked in horse serum as described in Materials and Methods and incubated with the selected primary antibody (pAkt), diluted in blocking buffer (based on the manufacturer's recommendations) overnight at 4°C with gentle rocking. After three washes with TBS/Triton, the slides were incubated with a Universal Secondary antibody for 1 hour at room temperature. After washes with TBS and TBS/Triton, the slides were incubated with 0.6% hydrogen peroxide for 30 minutes. The slides were washed once more with TBS and TBS/Triton and stable DAB was added to each spot on the slide for 15 to 20 minutes. Slides were then rinsed with water and mounted with a coverslip. (B) Logarithmically growing PC-3 cells were treated with 5 µg/ml Nexrutine or with solvent control for different periods (0, 3, 6, 12, and 24 hours) and whole-cell extracts were prepared. Western blotting was performed as described above for Figure 3C. The blot shown is a representative blot that was repeated three different times. (C) To measure levels of total andphosphorylated Akt using FACE-Akt kit, exponentially growing cells in 96-well plates were treated with different concentrations of Nexrutine. Cells were rapidly fixed and incubated with a primary antibody that recognizes either phosphorylated or total Akt. This was followed by incubation with secondary HRP-conjugated antibody followed by colorimetric development. Relative number of cells in each well was determined using crystal violet reagent for normalizing the data. The data shown here is a representative of three experiments and the values represent averages obtained from triplicate wells. (D) Subconfluent LNCaP cells were transfected with either pCMVMyrAkt (an activated form of Akt with the Src myristoylation signal fused in-frame to the c-Akt coding sequence) or control vector (pCMV) using Lipofectamin (Invitrogen) in triplicate dishes. Forty-eight hours after transfection, cells were treated with either 5 µg/ml Nexrutine or solvent control. Both floating and adherent cells were collected after 24 hours of treatment and assessed for cell viability by trypan blue exclusion assay. Data are averages ± S.D. of three independent experiments.
Figure 5
Nexrutine treatment reduces the levels of pCREB and CREB DNA-binding activity in LNCaP and PC-3 cells. Logarithmically growing LNCaP cells were treated with Nexrutine (0, 1, 2.5, and 5 µg/ml for 24 hours) or with solvent control and nuclear extracts were prepared. In the case of PC-3 cells, cells were treated with 5 µg/ml of Nexrutine for different periods (0, 3, 6, 12, and 24 hours). Western blotting was performed as described for Figure 4B. (A) Western blotting of LNCaP extracts. (B) Western blotting of extracts prepared from PC-3 cells. Nuclear extracts prepared from human fibroblast WI-38 cells stimulated with forskolin (CREB activator) was used as positive control (Std). (C) CREB DNA-binding activity was measured in LNCaP nuclear extracts by using TransAM CREB (Active Motif). Briefly, the control and Nexrutine-treated nuclear extracts were incubated with CREB consensus oligonucleotide that was immobilized in 96-well plates. A primary antibody specific for an epitope on the bound and active form of the CREB was then added followed by subsequent incubation with secondary antibody and developing solution. After this incubation with the developing solution, CREB activity was measured colorimetrically at 450 nm. Nuclear extracts prepared from human fibroblast WI-38 cells stimulated with forskolin (CREB activator) were used as positive control. For competition experiments, the wells containing immobilized oligo were preincubated with 100-fold molar excess of wild-type and mutant oligonucleotide for 30 min before addition of the nuclear extract. (D) CREB DNA-binding activity in PC-3 cells using TransAM CREB (Active Motif) as described above for (C). (E) Akt kinase activity in extracts prepared from PC-3 cells treated with Nexrutine (5 µg/ml) for different time intervals. Endogenous Akt was immunoprecipitated with Akt antibody. Kinase reaction was performed in the presence of cold ATP and GSK-3 substrate as per manufacturer's recommendations (Cell Signaling Technology). Phosphorylation of GSK-3 was measured by Western blotting using an anti-phospho GSK-3 antibody. The blot shown is a representative blot of two independent experiments.
Similar articles
- Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.
Lee CC, Chen CW, Yen HK, Lin YP, Lai CY, Wang JL, Groot OQ, Janssen SJ, Schwab JH, Hsu FM, Lin WH. Lee CC, et al. Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924 - Identification of a novel toxicophore in anti-cancer chemotherapeutics that targets mitochondrial respiratory complex I.
Stephenson ZA, Harvey RF, Pryde KR, Mistry S, Hardy RE, Serreli R, Chung I, Allen TE, Stoneley M, MacFarlane M, Fischer PM, Hirst J, Kellam B, Willis AE. Stephenson ZA, et al. Elife. 2020 May 20;9:e55845. doi: 10.7554/eLife.55845. Elife. 2020. PMID: 32432547 Free PMC article. - Ceftazidime with avibactam for treating severe aerobic Gram-negative bacterial infections: technology evaluation to inform a novel subscription-style payment model.
Harnan S, Kearns B, Scope A, Schmitt L, Jankovic D, Hamilton J, Srivastava T, Hill H, Ku CC, Ren S, Rothery C, Bojke L, Sculpher M, Woods B. Harnan S, et al. Health Technol Assess. 2024 Oct;28(73):1-230. doi: 10.3310/YAPL9347. Health Technol Assess. 2024. PMID: 39487661 Free PMC article. - Topical fluoride as a cause of dental fluorosis in children.
Wong MCM, Zhang R, Luo BW, Glenny AM, Worthington HV, Lo ECM. Wong MCM, et al. Cochrane Database Syst Rev. 2024 Jun 20;6(6):CD007693. doi: 10.1002/14651858.CD007693.pub3. Cochrane Database Syst Rev. 2024. PMID: 38899538 Review.
Cited by
- Histone Demethylase KDM5C Drives Prostate Cancer Progression by Promoting EMT.
Lemster AL, Sievers E, Pasternack H, Lazar-Karsten P, Klümper N, Sailer V, Offermann A, Brägelmann J, Perner S, Kirfel J. Lemster AL, et al. Cancers (Basel). 2022 Apr 8;14(8):1894. doi: 10.3390/cancers14081894. Cancers (Basel). 2022. PMID: 35454801 Free PMC article. - Palmatine inhibits growth and invasion in prostate cancer cell: Potential role for rpS6/NFκB/FLIP.
Hambright HG, Batth IS, Xie J, Ghosh R, Kumar AP. Hambright HG, et al. Mol Carcinog. 2015 Oct;54(10):1227-34. doi: 10.1002/mc.22192. Epub 2014 Jul 7. Mol Carcinog. 2015. PMID: 25043857 Free PMC article. - Genomic organization, alternative splicing and tissues expression of porcine CREB3L4 gene.
Qi YM, Lei T, Zhou L, Chen XD, Long QQ, Long H, Jin D, Gan L, Yang ZQ. Qi YM, et al. Mol Biol Rep. 2009 Sep;36(7):1881-8. doi: 10.1007/s11033-008-9394-1. Epub 2008 Nov 4. Mol Biol Rep. 2009. PMID: 18982425 - Evidence for the Possible Biological Significance of the igf-1 Gene Alternative Splicing in Prostate Cancer.
Philippou A, Armakolas A, Koutsilieris M. Philippou A, et al. Front Endocrinol (Lausanne). 2013 Mar 20;4:31. doi: 10.3389/fendo.2013.00031. eCollection 2013. Front Endocrinol (Lausanne). 2013. PMID: 23519101 Free PMC article. - Dietary administration of berberine or Phellodendron amurense extract inhibits cell cycle progression and lung tumorigenesis.
James MA, Fu H, Liu Y, Chen DR, You M. James MA, et al. Mol Carcinog. 2011 Jan;50(1):1-7. doi: 10.1002/mc.20690. Mol Carcinog. 2011. PMID: 21061266 Free PMC article.
References
- Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ. Cancer statistics. CA Cancer J Clin. 2004;54:8–29. - PubMed
- Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. - PubMed
- Park EJ, Pezzuto JM. Botanicals in cancer chemoprevention. Cancer Metastasis Rev. 2002;21:231–255. - PubMed
- Giovannucci E. Tomato products, lycopene, and prostate cancer: a review of the epidemiological literature. J Nutr. 2005;135:2030S–2031S. - PubMed
- Cohen JH, Kristal AR, Stanford JL. Fruits and vegetable intakes and prostate cancer risk. J Natl Cancer Inst. 2000;92:61–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 CA098744/CA/NCI NIH HHS/United States
- R21 CA098744-01A1/CA/NCI NIH HHS/United States
- P30 CA046934/CA/NCI NIH HHS/United States
- R21 CA 98744/CA/NCI NIH HHS/United States
- CA 46934-15S1/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials