Use of a nonmedicated dietary supplement correlates with increased prevalence of streptomycin-sulfa-tetracycline-resistant Escherichia coli on a dairy farm - PubMed (original) (raw)
Use of a nonmedicated dietary supplement correlates with increased prevalence of streptomycin-sulfa-tetracycline-resistant Escherichia coli on a dairy farm
Artashes R Khachatryan et al. Appl Environ Microbiol. 2006 Jul.
Abstract
We examined how a dietary supplement affects the prevalence of antibiotic-resistant Escherichia coli on a dairy farm in Washington State. Between 2001 and 2004 the prevalence of fecal E. coli strains resistant to streptomycin, sulfadiazine, and tetracycline (SSuT strains) declined from 59.2% to 26.1% in the calf population. In 2003 the dairy discontinued use of a dietary supplement, and we hypothesized that the decline in prevalence of SSuT strains was related to this change in management. To test this we established three treatments in which calves received no supplement, the dietary supplement with oxytetracycline, or the dietary supplement without oxytetracycline. Calves receiving either dietary supplement had a significantly higher prevalence of SSuT E. coli than the no-supplement control group (approximately 37% versus 20%, respectively; P = 0.03). Importantly, there was no evidence that oxytetracycline contributed to an increased prevalence of fecal SSuT E. coli. We compared the growth characteristics of SSuT and non-SSuT E. coli in LB broth enriched with either the complete dietary supplement or its individual constituents. Both the complete dietary supplement and its vitamin D component supported a significantly higher cell density of SSuT strains (P = 0.003 and P = 0.001, respectively). The dry milk and vitamin A components of the dietary supplement did not support different cell densities. These results were consistent with selection and maintenance of SSuT E. coli due to environmental components independent of antibiotic selection.
Figures
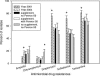
FIG. 1.
Frequency of antimicrobial drug resistance for all E. coli isolates shed from calves: year 2001 (n = 18 calves), year 2004 (n = 30), group receiving supplement without Pennox-50 (n = 9), group receiving supplement with Pennox-50 (n = 9), and group not receiving supplement or Pennox-50 (n = 9). + and *, statistically significant (P < 0.05 by Student's t test between years and repeated-measures ANOVA test for 2004 experiment, respectively); error bars indicate standard errors.
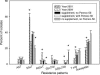
FIG. 2.
Frequency of antimicrobial drug resistance patterns for all E. coli isolates shed from calves: year 2001 (n = 18), year 2004 (n = 30), group receiving supplement without Pennox-50 (n = 9), group receiving supplement with Pennox-50 (n = 9), and group not receiving supplement or Pennox-50 (n = 9). + and *, statistically significant (P < 0.05 by Student's t test between years and repeated-measures ANOVA test for 2004 experiment, respectively). Resistance patterns are denoted by letters, where A is ampicillin, Ch is chloramphenicol, S is streptomycin, Su is sulfadiazine, and T is tetracycline. Susceptible isolates were susceptible to all above-mentioned antimicrobial drugs tested; error bars indicate standard errors.
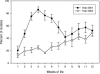
FIG. 3.
Distribution of SSuT E. coli isolates over weeks of life for all the calves for year 2001 (n = 18) and year 2004 (n = 30); error bars indicate standard errors.
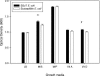
FIG. 4.
Absorbance values at 24 h of growth for SSuT and susceptible E. coli. LB, LB broth (six replicates for each resistotype); M/S, LB broth supplemented with dietary supplement (not containing oxytetracycline; three replicates for each resistotype); M/P, LB broth supplemented with milk powder (three replicates for each resistotype); Vit A, LB broth supplemented with vitamin A (three replicates for each resistotype); Vit D, LB broth supplemented with vitamin D (three replicates for each resistotype). Error bars indicate standard errors; *, statistically significant difference between SSuT and susceptible E. coli (P < 0.05 by Student's t test).
Similar articles
- Role of calf-adapted Escherichia coli in maintenance of antimicrobial drug resistance in dairy calves.
Khachatryan AR, Hancock DD, Besser TE, Call DR. Khachatryan AR, et al. Appl Environ Microbiol. 2004 Feb;70(2):752-7. doi: 10.1128/AEM.70.2.752-757.2004. Appl Environ Microbiol. 2004. PMID: 14766551 Free PMC article. - The streptomycin-sulfadiazine-tetracycline antimicrobial resistance element of calf-adapted Escherichia coli is widely distributed among isolates from Washington state cattle.
Khachatryan AR, Besser TE, Call DR. Khachatryan AR, et al. Appl Environ Microbiol. 2008 Jan;74(2):391-5. doi: 10.1128/AEM.01534-07. Epub 2007 Nov 26. Appl Environ Microbiol. 2008. PMID: 18039823 Free PMC article. - Changes in tetracycline susceptibility of enteric bacteria following switching to nonmedicated milk replacer for dairy calves.
Kaneene JB, Warnick LD, Bolin CA, Erskine RJ, May K, Miller R. Kaneene JB, et al. J Clin Microbiol. 2008 Jun;46(6):1968-77. doi: 10.1128/JCM.00169-08. Epub 2008 Apr 16. J Clin Microbiol. 2008. PMID: 18417664 Free PMC article. - Animal and farm influences on the dynamics of antibiotic resistance in faecal Escherichia coli in young dairy calves.
Berge AC, Atwill ER, Sischo WM. Berge AC, et al. Prev Vet Med. 2005 Jun 10;69(1-2):25-38. doi: 10.1016/j.prevetmed.2005.01.013. Epub 2005 Feb 26. Prev Vet Med. 2005. PMID: 15899294
Cited by
- The genetic diversity of commensal Escherichia coli strains isolated from non-antimicrobial treated pigs varies according to age group.
Ahmed S, Olsen JE, Herrero-Fresno A. Ahmed S, et al. PLoS One. 2017 May 30;12(5):e0178623. doi: 10.1371/journal.pone.0178623. eCollection 2017. PLoS One. 2017. PMID: 28558003 Free PMC article. - Food animals and antimicrobials: impacts on human health.
Marshall BM, Levy SB. Marshall BM, et al. Clin Microbiol Rev. 2011 Oct;24(4):718-33. doi: 10.1128/CMR.00002-11. Clin Microbiol Rev. 2011. PMID: 21976606 Free PMC article. Review. - Effect of preweaned dairy calf housing system on antimicrobial resistance in commensal Escherichia coli.
Pereira RV, Siler JD, Ng JC, Davis MA, Warnick LD. Pereira RV, et al. J Dairy Sci. 2014 Dec;97(12):7633-43. doi: 10.3168/jds.2014-8588. Epub 2014 Oct 11. J Dairy Sci. 2014. PMID: 25306277 Free PMC article. - Prevalence of Antimicrobial Resistance and Characteristics of Escherichia coli Isolates From Fecal and Manure Pit Samples on Dairy Farms in the Province of Québec, Canada.
Massé J, Lardé H, Fairbrother JM, Roy JP, Francoz D, Dufour S, Archambault M. Massé J, et al. Front Vet Sci. 2021 May 21;8:654125. doi: 10.3389/fvets.2021.654125. eCollection 2021. Front Vet Sci. 2021. PMID: 34095273 Free PMC article. - Antimicrobial resistance and prevalence of extended-spectrum beta-lactamase-producing Klebsiella species in East Tennessee dairy farms.
Gelalcha BD, Gelgie AE, Kerro Dego O. Gelalcha BD, et al. Microbiol Spectr. 2024 Oct 3;12(10):e0353723. doi: 10.1128/spectrum.03537-23. Epub 2024 Sep 6. Microbiol Spectr. 2024. PMID: 39240080 Free PMC article.
References
- Aarestrup, F. M., Y. Agerso, P. Gerner-Smidt, M. Madsen, and L. B. Jensen. 2000. Comparison of antimicrobial resistance phenotypes and resistance genes in Enterococcus faecalis and Enterococcus faecium from humans in the community, broilers, and pigs in Denmark. Diagn. Microbiol. Infect. Dis. 37:127-137. - PubMed
- Aarestrup, F. M., A. M. Seyfarth, H. D. Emborg, K. Pedersen, R. S. Hendriksen, and F. Bager. 2001. Effect of abolishment of the use of antimicrobial agents for growth promotion on occurrence of antimicrobial resistance in fecal enterococci from food animals in Denmark. Antimicrob. Agents Chemother. 45:2054-2059. - PMC - PubMed
- Anderson, K. L., T. G. Nagaraja, J. L. Morrill, P. G. Reddy, T. B. Avery, and N. V. Anderson. 1988. Performance and ruminal changes of early-weaned calves fed lasalocid. J. Anim. Sci. 66:806-813. - PubMed
- Andersson, D. I., and B. R. Levin. 1999. The biological cost of antibiotic resistance. Curr. Opin. Microbiol. 2:489-493. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical