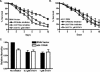The Caenorhabditis elegans ABL-1 tyrosine kinase is required for Shigella flexneri pathogenesis - PubMed (original) (raw)
The Caenorhabditis elegans ABL-1 tyrosine kinase is required for Shigella flexneri pathogenesis
Elizabeth A Burton et al. Appl Environ Microbiol. 2006 Jul.
Abstract
Shigellosis is a diarrheal disease caused by the gram-negative bacterium Shigella flexneri. Following ingestion of the bacterium, S. flexneri interferes with innate immunity, establishes an infection within the human colon, and initiates an inflammatory response that results in destruction of the tissue lining the gut. Examination of host cell factors required for S. flexneri pathogenesis in vivo has proven difficult due to limited host susceptibility. Here we report the development of a pathogenesis system that involves the use of Caenorhabditis elegans as a model organism to study S. flexneri virulence determinants and host molecules required for pathogenesis. We show that S. flexneri-mediated killing of C. elegans correlates with bacterial accumulation in the intestinal tract of the animal. The S. flexneri virulence plasmid, which encodes a type III secretory system as well as various virulence determinants crucial for pathogenesis in mammalian systems, was found to be required for maximal C. elegans killing. Additionally, we demonstrate that ABL-1, the C. elegans homolog of the mammalian c-Abl nonreceptor tyrosine kinase ABL1, is required for S. flexneri pathogenesis in nematodes. These data demonstrate the feasibility of using C. elegans to study S. flexneri pathogenesis in vivo and provide insight into host factors that contribute to S. flexneri pathogenesis.
Figures
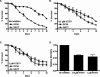
FIG. 1.
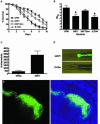
S. flexneri kills C. elegans and accumulates in the nematode intestine. (A) Young adult hermaphrodite N2 nematodes were fed on lawns of either E. coli OP50, S. flexneri 2457T, S. flexneri 2457TΔinv, or S. enterica serovar Typhimurium SL1344 at 25°C, and nematodes were scored daily for survival. The survival curve represents data from 5 independent experiments, each using 20 nematodes. (B) The time required for nematodes to die (TD50) was calculated using the survival data in panel A, representing data from five independent experiments. Asterisks indicate significant differences compared to data for OP50 (P < 0.05). (C) Young adult nematodes were fed on lawns of either E. coli DH5α or S. flexneri 2457T expressing GFP for 48 h and mechanically disrupted. Diluted lysates were plated on LB-ampicillin plates, and colonies were quantified in order to calculate S. flexneri cells associated with individual nematodes. These data represent 3 independent experiments, each using 20 nematodes. (D) Nematodes fed either E. coli DH5α or S. flexneri 2457T expressing GFP for 48 h were analyzed using a fluorescence stereomicroscope. (E) Confocal images show the anterior intestine of an animal fed S. flexneri 2457T expressing GFP for 48 h.
FIG. 2.
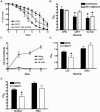
The mechanism of _S. flexneri_-mediated C. elegans killing is distinct from that of S. enterica and P. aeruginosa. (A) Young adult hermaphrodite nematodes were fed on lawns of either E. coli OP50 (squares) or S. flexneri 2457T (closed circles) continuously or fed on S. flexneri 2457T for 24 h and then transferred to OP50 for the duration of the assay (open circles). Nematodes were scored daily for survival. The survival curve represents data from three independent experiments, each using 20 nematodes. (B) The time required for nematodes to die (TD50) was calculated from the survival data in panel A, representing data from three independent experiments. Asterisks indicate significant differences, compared to results for OP50 (P < 0.05). (C) Young adult hermaphrodite nematodes were fed on E. coli DH5α-GFP (squares), S. flexneri 2457T-GFP (circles), or S. enterica serovar Typhimurium SL1344-GFP (asterisks) for 24 h and then transferred to lawns of E. coli OP50. Nematodes were removed every 24 h and mechanically disrupted to release internalized bacteria. Diluted lysates were plated on LB-ampicillin plates, and colonies were scored in order to quantify S. flexneri cells associated with individual nematodes. (D) Young adult hermaphrodites were fed on either live or UV-killed E. coli OP50 or S. flexneri 2457T and scored daily for survival. The time for nematodes to die (TD50) was calculated from the survival data. Asterisks indicate significant differences, compared to results for OP50 (P < 0.05). (E) Young adult nematodes were fed on either E. coli OP50 or S. flexneri 2457T or on E. coli OP50 plated on agar plates incubated with E. coli OP50 or S. flexneri 2457T separated by a 0.45-μm filter. The nematodes were scored daily for survival, and the time required for nematodes to die (TD50) was calculated from the survival data. Asterisks indicate significant differences, compared to results for OP50 (P < 0.05).
FIG. 3.
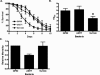
ABL-1 mutant nematodes are resistant to _S. flexneri_-mediated C. elegans killing. (A) Young adult hermaphrodite abl-1(ok171) nematodes were fed on lawns of either E. coli OP50, S. flexneri 2457T, or S. enterica serovar Typhimurium SL1344 at 25°C, and nematodes were scored daily for survival. The survival curve represents data from 5 independent experiments, each using 20 nematodes. These experiments also included analysis of N2 nematodes, shown in Fig. 1. (B) The time required for nematodes to die (TD50) was calculated from the survival data in panel A, representing data from three independent experiments. The asterisk indicates significant difference compared to results for OP50 (P < 0.05). (C) The relative mortality was calculated using the TD50 values for N2 and abl-1(ok171) nematodes in order to normalize the survival rates of the nematodes grown on pathogens compared to that of nematodes fed on E. coli OP50 (2).
FIG. 4.
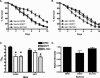
ABL-1 knockdown nematodes are resistant to _S. flexneri-_mediated C. elegans killing. (A and B) Vector (panel A) or abl-1 RNAi (panel B) young adult hermaphrodite nematodes were fed on lawns of either E. coli OP50, S. flexneri 2457T, or S. enterica serovar Typhimurium SL1344 at 25°C, and nematodes were scored daily for survival. The survival curve represents data from 9 independent experiments, each using 20 nematodes. (C) The time for nematodes to die (TD50) was calculated from the survival data in panels A and B. Asterisks indicate significant differences, compared to results for OP50 (P < 0.05), representing data from nine independent experiments. (D) The relative mortality was calculated using the TD50 values for the vector and abl-1 RNAi nematodes in order to normalize the survival rates of the nematodes grown on pathogens compared to that of nematodes fed on E. coli OP50 (2).
FIG. 5.
Nematodes treated with STI571 are resistant to _S. flexneri_-mediated C. elegans killing. (A to C) Young adult hermaphrodite nematodes were fed on lawns of either E. coli OP50 or S. flexneri 2457T at 25°C in the presence of PBS (A) or 0.5 μM (B) or 1 μM (C) STI571, and nematodes were scored daily for survival. The survival curve represents data from 3 independent experiments, each using 20 nematodes. (D) The relative mortality was calculated using the TD50 values (representing data from three independent experiments) for the PBS and STI571-treated nematodes, in order to normalize the survival rates of the nematodes grown on S. flexneri 2457T compared to that of nematodes fed on E. coli OP50 (2).
FIG. 6.
STI571 is targeting ABL-1 during S. flexneri infection. (A and B) Vector (A) or abl-1 RNAi (B) young adult hermaphrodite nematodes were fed on lawns of either E. coli OP50 or S. flexneri 2457T at 25°C in the presence of PBS (filled symbols) or 1 μM STI571 (open symbols), and nematodes were scored daily for survival. The survival curve represents data from 4 independent experiments, each using 20 nematodes. (C) The relative mortality was calculated using the TD50 values (representing data from four independent experiments) for the PBS and STI571-treated nematodes, in order to normalize the survival rates of the nematodes grown on S. flexneri 2457T compared to that of nematodes fed on E. coli OP50 (2).
References
- Aballay, A., and F. M. Ausubel. 2002. Caenorhabditis elegans as a host for the study of host-pathogen interactions. Curr. Opin. Microbiol. 5:97-101. - PubMed
- Aballay, A., P. Yorgey, and F. M. Ausubel. 2000. Salmonella typhimurium proliferates and establishes a persistent infection in the intestine of Caenorhabditis elegans. Curr. Biol. 10:1539-1542. - PubMed
- Alegado, R. A., M. C. Campbell, W. C. Chen, S. S. Slutz, and M. W. Tan. 2003. Characterization of mediators of microbial virulence and innate immunity using the Caenorhabditis elegans host-pathogen model. Cell. Microbiol. 5:435-444. - PubMed
- Bidawid, S. P., J. F. Edeson, J. Ibrahim, and R. M. Matossian. 1978. The role of non-biting flies in the transmission of enteric pathogens (Salmonella species and Shigella species) in Beirut, Lebanon. Ann. Trop. Med. Parasitol. 72:117-121. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM070977/GM/NIGMS NIH HHS/United States
- R01 GM062375/GM/NIGMS NIH HHS/United States
- R21 AI065641/AI/NIAID NIH HHS/United States
- R37 GM070977/GM/NIGMS NIH HHS/United States
- GM62375/GM/NIGMS NIH HHS/United States
- GM70977/GM/NIGMS NIH HHS/United States
- CA009111-27/CA/NCI NIH HHS/United States
- AI065641/AI/NIAID NIH HHS/United States
- R01 CA070940/CA/NCI NIH HHS/United States
- CA70940/CA/NCI NIH HHS/United States
- T32 CA009111/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous