AMP-activated protein kinase signaling in metabolic regulation - PubMed (original) (raw)
Review
AMP-activated protein kinase signaling in metabolic regulation
Yun Chau Long et al. J Clin Invest. 2006 Jul.
Abstract
AMP-activated protein kinase (AMPK) is an energy sensor that regulates cellular metabolism. When activated by a deficit in nutrient status, AMPK stimulates glucose uptake and lipid oxidation to produce energy, while turning off energy-consuming processes including glucose and lipid production to restore energy balance. AMPK controls whole-body glucose homeostasis by regulating metabolism in multiple peripheral tissues, such as skeletal muscle, liver, adipose tissues, and pancreatic beta cells--key tissues in the pathogenesis of type 2 diabetes. By responding to diverse hormonal signals including leptin and adiponectin, AMPK serves as an intertissue signal integrator among peripheral tissues, as well as the hypothalamus, in the control of whole-body energy balance.
Figures
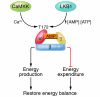
Figure 1. Structure and regulation of AMPK.
AMPK is a heterotrimeric complex consisting of an α, a β, and a γ subunit and is activated by the upstream kinases CaMKK and LKB1 via phosphorylation of threonine residue 172. Activation of AMPK by CaMKK and LKB1 is dependent on the intracellular calcium and AMP/ATP ratio, respectively. Generally, AMPK restores energy balance by activating processes that produce energy (e.g., lipid oxidation and glucose uptake) while inhibiting those that consume energy (e.g., protein synthesis).
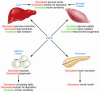
Figure 2. Role of AMPK in the regulation of whole-body glucose homeostasis.
Activation of AMPK turns on ATP-generating processes, while switching off ATP-consuming processes. In skeletal muscle, acute activation of AMPK increases glucose uptake and lipid oxidation, while chronic activation of AMPK is associated with mitochondrial biogenesis. Activation of AMPK inhibits glucose and lipid synthesis in the liver but increases lipid oxidation. Lipolysis and lipogenesis in adipose tissue are also reduced by AMPK activation. Collectively, activation of AMPK in skeletal muscle, liver, and adipose tissue results in a favorable metabolic milieu for the prevention or treatment of T2D, i.e., decreased circulating glucose, reduced plasma lipid, and ectopic fat accumulation, as well as enhanced insulin sensitivity. Activation of pancreatic AMPK is associated with decreased insulin secretion, likely a protective measure to prevent hypoglycemia during food deprivation, although this effect needs to be considered in pharmaceutical targeting of AMPK for the treatment of T2D.
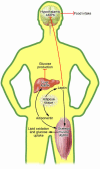
Figure 3. Integration of intertissue signaling by AMPK.
Adipocyte-derived leptin reduces food intake though inhibition of hypothalamic AMPK. Leptin increases skeletal muscle fatty acid oxidation via direct activation of AMPK and the hypothalamus–sympathetic nervous system axis. The involvement of hypothalamic AMPK in leptin-induced skeletal muscle lipid oxidation and glucose uptake is unknown. Adiponectin is secreted by adipose tissue and increases skeletal muscle lipid oxidation and glucose uptake, as well as suppressing liver glucose production by activating AMPK.
Similar articles
- The AMP-activated protein kinase: more than an energy sensor.
Hue L, Rider MH. Hue L, et al. Essays Biochem. 2007;43:121-37. doi: 10.1042/BSE0430121. Essays Biochem. 2007. PMID: 17705797 Review. - AMP-activated protein kinase and the metabolic syndrome.
Fryer LG, Carling D. Fryer LG, et al. Biochem Soc Trans. 2005 Apr;33(Pt 2):362-6. doi: 10.1042/BST0330362. Biochem Soc Trans. 2005. PMID: 15787607 Review. - Characterization of the AMP-activated protein kinase pathway in chickens.
Proszkowiec-Weglarz M, Richards MP, Ramachandran R, McMurtry JP. Proszkowiec-Weglarz M, et al. Comp Biochem Physiol B Biochem Mol Biol. 2006 Jan;143(1):92-106. doi: 10.1016/j.cbpb.2005.10.009. Epub 2005 Dec 15. Comp Biochem Physiol B Biochem Mol Biol. 2006. PMID: 16343965 - [AMP-activated protein kinase--the key role in metabolic regulation].
Jarzyna R. Jarzyna R. Postepy Biochem. 2006;52(3):283-8. Postepy Biochem. 2006. PMID: 17201063 Review. Polish. - AMPK-dependent hormonal regulation of whole-body energy metabolism.
Dzamko NL, Steinberg GR. Dzamko NL, et al. Acta Physiol (Oxf). 2009 May;196(1):115-27. doi: 10.1111/j.1748-1716.2009.01969.x. Epub 2009 Feb 19. Acta Physiol (Oxf). 2009. PMID: 19245657 Review.
Cited by
- Nrf2 modulates the benefits of evening exercise in type 2 diabetes.
Fasipe B, Laher I. Fasipe B, et al. Sports Med Health Sci. 2023 Sep 9;5(4):251-258. doi: 10.1016/j.smhs.2023.09.001. eCollection 2023 Dec. Sports Med Health Sci. 2023. PMID: 38314046 Free PMC article. Review. - Inactivation of MARK4, an AMP-activated protein kinase (AMPK)-related kinase, leads to insulin hypersensitivity and resistance to diet-induced obesity.
Sun C, Tian L, Nie J, Zhang H, Han X, Shi Y. Sun C, et al. J Biol Chem. 2012 Nov 2;287(45):38305-15. doi: 10.1074/jbc.M112.388934. Epub 2012 Sep 19. J Biol Chem. 2012. PMID: 22992738 Free PMC article. - Prenatal metformin exposure in mice programs the metabolic phenotype of the offspring during a high fat diet at adulthood.
Salomäki H, Vähätalo LH, Laurila K, Jäppinen NT, Penttinen AM, Ailanen L, Ilyasizadeh J, Pesonen U, Koulu M. Salomäki H, et al. PLoS One. 2013;8(2):e56594. doi: 10.1371/journal.pone.0056594. Epub 2013 Feb 15. PLoS One. 2013. PMID: 23457588 Free PMC article. - Glucocorticoid Receptor Alpha Targets SLC2A4 to Regulate Protein Synthesis and Breakdown in Porcine Skeletal Muscle Cells.
Du XL, Xu WJ, Shi JL, Guo K, Guo CT, Zheng R, Jiang SW, Chai J. Du XL, et al. Biomolecules. 2021 May 12;11(5):721. doi: 10.3390/biom11050721. Biomolecules. 2021. PMID: 34065777 Free PMC article. - AMP-activated kinase restricts Rift Valley fever virus infection by inhibiting fatty acid synthesis.
Moser TS, Schieffer D, Cherry S. Moser TS, et al. PLoS Pathog. 2012;8(4):e1002661. doi: 10.1371/journal.ppat.1002661. Epub 2012 Apr 19. PLoS Pathog. 2012. PMID: 22532801 Free PMC article.
References
- Klein S., et al. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies. A statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Diabetes Care. 2004;27:2067–2073. - PubMed
- Wing R.R., et al. Behavioral science research in diabetes: lifestyle changes related to obesity, eating behavior, and physical activity. Diabetes Care. 2001;24:117–123. - PubMed
- Hardie D.G. The AMP-activated protein kinase pathway: new players upstream and downstream. J. Cell Sci. 2004;117:5479–5487. - PubMed
- Carling D. The AMP-activated protein kinase cascade: a unifying system for energy control. . Trends Biochem. Sci. 2004;29:18–24. - PubMed
- Kemp B.E., et al. AMP-activated protein kinase, super metabolic regulator. Biochem. Soc. Trans. 2003;31:162–168. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources