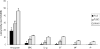Alzheimer disease with amygdala Lewy bodies: a distinct form of alpha-synucleinopathy - PubMed (original) (raw)
Alzheimer disease with amygdala Lewy bodies: a distinct form of alpha-synucleinopathy
Hirotake Uchikado et al. J Neuropathol Exp Neurol. 2006 Jul.
Abstract
Lewy bodies (LBs) are alpha-synuclein-immunoreactive neuronal inclusions with a predilection for specific cortical and subcortical regions, including the amygdala. In this study, the presence of LBs was assessed in 347 cases of Alzheimer disease (AD). In 87 cases, LB pathology was diagnostic of brainstem (n=3), transitional (n=32), or diffuse (n=52) Lewy body disease (LBD). The remaining 260 cases of AD were screened for amygdala LBs (AD/ALB) and 62 (24%) cases were found. If AD/LBD cases are included, LBs were detected in 149 (43%) cases of AD. The presence alpha-synuclein pathology was assessed in multiple brain regions of the 62 cases of AD/ALB and 57 randomly selected cases of AD, and only sparse alpha-synuclein pathology was detected in both. The burden of alpha-synuclein pathology in brainstem nuclei, amygdala, and neocortex was significant lower in AD/ALB than in AD/LBD. In comparison to AD/LBD, AD/ALB did not differ in age at death, disease duration, male-to-female ratio, brain weight, Braak neurofibrillary tangle stage, average senile plaque density, or apolipoprotein E epsilon4 allele frequency. The results suggest that AD/ALB is pathologically different from AD/LBD, suggesting that it is a neuropathologically distinct and isolated alpha-synucleinopathy.
Figures
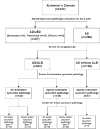
FIGURE 1
A flow chart of the study design and classification of cases stemming from standardized neuropathologic evaluation to diagnose Alzheimer disease (AD) or AD with concurrent Lewy body disease, followed by screening of the amygdala section for α-synuclein pathology in all AD cases. For those cases with any amygdala α-synuclein pathology, brainstem sections and limbic areas (entorhinal and cingulate gyrus) are screened for additional α-synuclein pathology. A randomly selected series of AD cases without amygdala α-synuclein pathology are processed in the same manner.
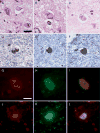
FIGURE 2
(A–C) Range of morphologies of amygdala Lewy bodies (LBs) with hematoxylin and eosin. Only exceptionally do amygdala LBs have dense hyaline appearance (C); more often, they are pleomorphic lesions indistinguishable from neurofibrillary tangles (NFTs) (A, B). Double immunostaining with α-synuclein (brown) and phospho-tau (blue) shows a range of morphologies as well. The α-synuclein-positive lesions may be separate from tau-positive NFTs (D) or variably intermingled within the same neuron (E, F). Double immunofluorescence for α-synuclein (red) and tau (green). In the merged images (I, L), colocalization appears yellow. The neuron in (G–I) shows intermingling of 2 distinct types of filaments within the same neuron, whereas the neuron in (J–L) shows an α-synuclein-immunoreactive, Lewy-like inclusion surrounded by a tau-immunoreactive NFT. Scale bars = (A–F) 25 μm; (D–I) 10 μm.
FIGURE 3
Immunoelectron microscopy with double labeling for phospho-tau (18-nm gold particles) and α-synuclein (5-nm gold particles) in a case of Alzheimer disease with amygdala Lewy bodies. (A) At low magnification, a neuron with separate filamentous aggregates (right) is distinguished from the neuron with intermingled filaments (left). L, lipofuscin. Boxed areas are enlarged at the same magnification in (B) and (C). (B) In the intermingled area, tau filaments (arrowheads) have the morphology of paired helical filaments (P) or straight filaments (S). α-synuclein is localized to granulofilamentous aggregates, arrows). (C) Tightly packed tau filaments (arrowheads) are separate from α-synuclein-positive granulofilamentous aggregates (arrows). The α-synuclein filaments are thinner than tau filaments. Scale bars = (A) 1 μm; (B, C) 300 nm.
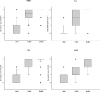
FIGURE 4
Comparison of α-synuclein score in subcortical regions vulnerable to α-synuclein pathology (DMN, LC, SN, and nbM) for AD/ALB, AD/TLBD, and AD/DLBD. The boxes show median and 25th and 75th percentiles with whisker plots showing 10th and 90th percentiles. The outliers are shown as filled circles. (Median scores for DMN, LC, SN, and nbM are, respectively: AD/ALB 0, 0, 0, 0; AD/TLBD 2.4, 1.7, 2.3, 1.4; and AD/DLBD 2.9, 2.9, 2.8, 2.1.) The α-synuclein scores in DMN, LC, SN and nbM are significantly less in AD/ALB than in AD/TLBD and AD/DLBD (*, p < 0.05). The α-synuclein scores in DMN, LC, SN, and nbM are significantly less in AD/TLBD than in AD/DLBD (#, p < 0.05). DMN, dorsal motor nucleus; nbM, nucleus of Meynert; AD/ALB, Alzheimer disease with amygdala Lewy bodies; AD/TLBD, Alzheimer disease with transitional Lewy body disease; AD/DLBD, Alzheimer disease with diffuse Lewy body disease.
FIGURE 5
Comparison of Lewy body (LB) density in amygdala and cortical regions for AD/ALB, AD/TLBD and AD/DLBD. Bar charts show mean and error bars show standard errors of the mean. The LB density in the amygdala was significantly less (*) in AD/ALB than in AD/TLBD and AD/DLBD. The LB density in the limbic cortices (ERC and Cing) and ST was significantly less (*) in AD/ALB than in AD/TLBD and AD/DLBD. The LB density in the limbic cortices and neocortical regions (ST, MF, and IP) was significantly less (#) in AD/TLBD than AD/DLBD. AD/ALB, Alzheimer disease with amygdala Lewy bodies; AD/TLBD, Alzheimer disease with transitional Lewy body disease; AD/DLBD, Alzheimer disease with diffuse Lewy body disease; ERC, entorhinal cortex; Cing, anterior cingulate gyrus; ST, superior temporal gyrus; MF, middle frontal gyrus; IP, inferior parietal gyrus.
FIGURE 6
(A) Neuronal population is well preserved in the substantia nigra with hematoxylin and eosin stain. (B) A few Lewy neurites (arrows) are found in the substantia nigra with α-synuclein immunostaining. (C) Neuronal loss with Lewy bodies (LBs) is found in the substantia nigra. Higher magnification (upper left corner, arrows) shows brainstem type LBs clearly. (D) Large amounts of LBs and Lewy neurites are found in the substantia nigra with α-synuclein immunostaining. (A, B) Alzheimer disease with amygdala Lewy bodies case, (C, D) Alzheimer disease with diffuse Lewy body disease case. Scale bars = (A–D) 50 μm.
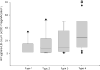
FIGURE 7
Average amygdala Lewy body counts in 4 subtypes of Alzheimer disease with amygdala Lewy bodies. The boxes show median and 25th and 75th percentiles with whisker plots showing 10th and 90th percentiles. The outliers are shown as filled circles. Type 1 cases have no α-synuclein pathology in the brainstem and limbic cortex. Type 2 cases have α-synuclein pathology only in the brainstem. Type 3 cases have α-synuclein pathology only in the limbic cortex. Type 4 cases have α-synuclein pathology in the brainstem and limbic cortex. #, Type 4 significantly different from type 1. LB, Lewy body.
Similar articles
- Lewy body-related alpha-synucleinopathy in the aged human brain.
Jellinger KA. Jellinger KA. J Neural Transm (Vienna). 2004 Oct;111(10-11):1219-35. doi: 10.1007/s00702-004-0138-7. Epub 2004 Apr 2. J Neural Transm (Vienna). 2004. PMID: 15480835 - Unique α-synuclein pathology within the amygdala in Lewy body dementia: implications for disease initiation and progression.
Sorrentino ZA, Goodwin MS, Riffe CJ, Dhillon JS, Xia Y, Gorion KM, Vijayaraghavan N, McFarland KN, Golbe LI, Yachnis AT, Giasson BI. Sorrentino ZA, et al. Acta Neuropathol Commun. 2019 Sep 2;7(1):142. doi: 10.1186/s40478-019-0787-2. Acta Neuropathol Commun. 2019. PMID: 31477175 Free PMC article. - Lewy bodies in progressive supranuclear palsy represent an independent disease process.
Uchikado H, DelleDonne A, Ahmed Z, Dickson DW. Uchikado H, et al. J Neuropathol Exp Neurol. 2006 Apr;65(4):387-95. doi: 10.1097/01.jnen.0000218449.17073.43. J Neuropathol Exp Neurol. 2006. PMID: 16691119 - Formation and development of Lewy pathology: a critical update.
Jellinger KA. Jellinger KA. J Neurol. 2009 Aug;256 Suppl 3:270-9. doi: 10.1007/s00415-009-5243-y. J Neurol. 2009. PMID: 19711116 Review. - Neuropathology of Lewy body disease: Clinicopathological crosstalk between typical and atypical cases.
Kon T, Tomiyama M, Wakabayashi K. Kon T, et al. Neuropathology. 2020 Feb;40(1):30-39. doi: 10.1111/neup.12597. Epub 2019 Sep 9. Neuropathology. 2020. PMID: 31498507 Review.
Cited by
- 100 years of Lewy pathology.
Goedert M, Spillantini MG, Del Tredici K, Braak H. Goedert M, et al. Nat Rev Neurol. 2013 Jan;9(1):13-24. doi: 10.1038/nrneurol.2012.242. Epub 2012 Nov 27. Nat Rev Neurol. 2013. PMID: 23183883 - Comparison of symptomatic and asymptomatic persons with Alzheimer disease neuropathology.
Monsell SE, Mock C, Roe CM, Ghoshal N, Morris JC, Cairns NJ, Kukull W. Monsell SE, et al. Neurology. 2013 Jun 4;80(23):2121-9. doi: 10.1212/WNL.0b013e318295d7a1. Epub 2013 May 3. Neurology. 2013. PMID: 23645594 Free PMC article. - Parkinson's disease and parkinsonism: neuropathology.
Dickson DW. Dickson DW. Cold Spring Harb Perspect Med. 2012 Aug 1;2(8):a009258. doi: 10.1101/cshperspect.a009258. Cold Spring Harb Perspect Med. 2012. PMID: 22908195 Free PMC article. Review. - Retrospective Evaluation of Neuropathologic Proxies of the Minimal Atrophy Subtype Compared With Corticolimbic Alzheimer Disease Subtypes.
Boon BDC, Labuzan SA, Peng Z, Matchett BJ, Kouri N, Hinkle KM, Lachner C, Ross OA, Ertekin-Taner N, Carter RE, Ferman TJ, Duara R, Dickson DW, Graff-Radford NR, Murray ME. Boon BDC, et al. Neurology. 2023 Oct 3;101(14):e1412-e1423. doi: 10.1212/WNL.0000000000207685. Epub 2023 Aug 14. Neurology. 2023. PMID: 37580158 Free PMC article. - Empiric refinement of the pathologic assessment of Lewy-related pathology in the dementia patient.
Leverenz JB, Hamilton R, Tsuang DW, Schantz A, Vavrek D, Larson EB, Kukull WA, Lopez O, Galasko D, Masliah E, Kaye J, Woltjer R, Clark C, Trojanowski JQ, Montine TJ. Leverenz JB, et al. Brain Pathol. 2008 Apr;18(2):220-4. doi: 10.1111/j.1750-3639.2007.00117.x. Epub 2008 Jan 29. Brain Pathol. 2008. PMID: 18241240 Free PMC article.
References
- McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): Report of the consortium on DLB international workshop. Neurology. 1996;47:1113–24. - PubMed
- Dickson DW, Crystal HA, Mattiace LA, et al. Identification of normal and pathological aging in prospectively studied nondemented elderly humans. Neurobiol Aging. 1992;13:179–89. - PubMed
- Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:1095–97. - PubMed
- Hansen L, Salmon D, Galasko D, et al. The Lewy body variant of Alzheimer’s disease: a clinical and pathologic entity. Neurology. 1990;40:1–8. - PubMed
- Mikolaenko I, Pletnikova O, Kawas CH, et al. Alpha-synuclein lesions in normal aging, Parkinson disease, and Alzheimer disease: evidence from the Baltimore Longitudinal Study of Aging (BLSA) J Neuropathol Exp Neurol. 2005;64:156–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01-AG17216/AG/NIA NIH HHS/United States
- P50 NS040256/NS/NINDS NIH HHS/United States
- P50 AG016574/AG/NIA NIH HHS/United States
- P01-AG14449/AG/NIA NIH HHS/United States
- P50-AG25711/AG/NIA NIH HHS/United States
- P50-AG16574/AG/NIA NIH HHS/United States
- P01 AG014449/AG/NIA NIH HHS/United States
- P50-NS40256/NS/NINDS NIH HHS/United States
- P01 AG003949/AG/NIA NIH HHS/United States
- P01-AG03949/AG/NIA NIH HHS/United States
- P50 AG025711/AG/NIA NIH HHS/United States
- P01 AG017216/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous