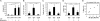Regulation of lymph node vascular growth by dendritic cells - PubMed (original) (raw)
Regulation of lymph node vascular growth by dendritic cells
Brian Webster et al. J Exp Med. 2006.
Abstract
Lymph nodes grow rapidly and robustly at the initiation of an immune response, and this growth is accompanied by growth of the blood vessels. Although the vessels are critical for supplying nutrients and for controlling cell trafficking, the regulation of lymph node vascular growth is not well understood. We show that lymph node endothelial cells begin to proliferate within 2 d of immunization and undergo a corresponding expansion in cell numbers. Endothelial cell proliferation is dependent on CD11c+ dendritic cells (DCs), and the subcutaneous injection of DCs is sufficient to trigger endothelial cell proliferation and growth. Lymph node endothelial cell proliferation is dependent on vascular endothelial growth factor (VEGF), and DCs are associated with increased lymph node VEGF levels. DC-induced endothelial cell proliferation and increased VEGF levels are mediated by DC-induced recruitment of blood-borne cells. Vascular growth in the draining lymph node includes the growth of high endothelial venule endothelial cells and is functionally associated with increased cell entry into the lymph node. Collectively, our results suggest a scenario whereby endothelial cell expansion in the draining lymph node is induced by DCs as part of a program that optimizes the microenvironment for the ensuing immune response.
Figures
Figure 1.
Growth of vascularity and of endothelial cells in the draining lymph node. Mice were immunized with OVA/CFA on the upper back and killed at the indicated times. (A) Total cell counts of draining brachial and nondraining inguinal (Ing) lymph nodes. (B) Vascularity of brachial lymph nodes at day 5. (A and B) n = 3 mice. (C) FACS plot showing the CD45negCD31hi endothelial cell population (R1). (D) Endothelial cell count in the same lymph nodes as in A. (E) Endothelial cell (EC) proliferation in brachial lymph nodes over the preceding 48 h at days 2 and 5 as measured by the percentage of endothelial cells that incorporated BrdU. (F) Endothelial cell proliferation in brachial lymph nodes over the preceding 24 h on days 1, 2, and 3. (E and F) Each symbol represents one mouse, and results are representative of experiments performed four times. (G) Immunohistochemical stain for PNAd (blue), BrdU (pink), and CD45 (brown) in a day 3 draining lymph node to show BrdU uptake by PNAd+ HEV endothelial cells. (H) Percentage of HEVs in brachial lymph nodes that have at least one BrdU+PNAD+ cell. n ≥ 4 mice for each time point. (A, B, D, and H) *, P < 0.05; **, P < 0.01 with the Student's t test compared with PBS-injected controls. (A, D, E, F, and H) Closed symbols represent samples from immunized mice, and open symbols represent samples from PBS-injected control mice. Error bars represent SD.
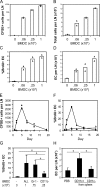
Figure 2.
CD11c+ cells are important for lymph node endothelial cell proliferation. (A) Endothelial cell proliferation on day 2 in wild-type or CD11c-DTR (DTR) mice upon immunization with OVA/CFA. n = 3 mice for each condition. *, P < 0.01; P = 0.76 for immunized wild-type versus immunized DTR mice. (B–D) Effects of DT treatment in immunized mice on CD11c+MHCII+ DCs per lymph node (B), lymph node size (C), and endothelial cell (EC) proliferation (D). n = 5 mice for each condition. (E) Plot of endothelial cell proliferation as a function of the number of CD11c+MHCII+ DCs remaining in draining lymph nodes of the DT-treated CD11c-DTR mice. (B–D) *, P < 0.03; **, P < 0.004 with the Student's t test. Error bars represent SD.
Figure 3.
DCs induce endothelial cell proliferation. (A–D) Indicated numbers of CFSE-labeled BMDCs were injected into the footpad, and mice were examined at day 5. Number of CFSE+ BMDCs (A), number of total cells (B), endothelial cell (EC) proliferation (C), and number of endothelial cells (D) in draining lymph nodes. Each symbol represents one mouse. Bars represent the mean values. (E and F) Number of CFSE+ BMDCs (E) and endothelial cell BrdU uptake (F) in draining lymph nodes over time after the injection of 106 BMDCs. Each symbol represents one mouse. Closed circles represent draining popliteal lymph nodes, and open triangles represent nondraining brachial lymph nodes from the same mice. (A–F) Results are representative of at least three separate experiments. (G) Endothelial cell BrdU uptake upon injection of unseparated BMDCs (All), granulocyte-depleted BMDCs (Gr-1−), or DC-depleted BMDCs (CD11c−). n ≥ 3 mice for each condition. *, P < 0.05 with the Student's t test. (H) Endothelial cell count at day 5 in draining popliteal lymph nodes of mice injected with PBS or with 106 CD11c+ or CD11c− cells isolated from the spleen. n ≥ 8. *, P < 0.00005 with the Student's t test. Error bars represent SD.
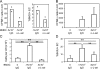
Figure 4.
DC-induced endothelial cell proliferation in RAG1−/− mice. BMDCs were injected into wild-type or RAG1−/− recipients and examined on day 2. (A and B) Lymph node cellularity in wild-type (A) and RAG1−/− (B) mice. (C) Number of CFSE+ cells in draining lymph nodes. (D) Endothelial cell (EC) BrdU uptake. n ≥ 6 mice for each condition. *, P < 0.05; **, P < 0.01 with the Student's t test. Error bars represent SD.
Figure 5.
Role of cell recruitment in endothelial cell proliferation. Indicated numbers of CFSE+ BMDCs were injected into anti–L-selectin–(α-L-sel) or control antibody (IgG)-treated recipients and examined on day 2. (A) CFSE+ BMDC accumulation (left) and endothelial cell BrdU uptake (right) upon injection of equal numbers of BMDCs. Each symbol represents one mouse, and data are representative of three similar experiments. (B–D) BMDC numbers were adjusted to obtain similar levels of BMDC accumulation in control and anti–L-selectin–treated mice. CFSE+ BMDC accumulation (B), lymph node cell counts (C), and endothelial cell (EC) BrdU uptake (D) in draining lymph nodes. n ≥ 9 mice for each condition. Bars represent the mean values. **, P < 0.007. Error bars represent SD.
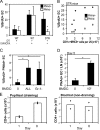
Figure 6.
HEV endothelial cell proliferation and increased lymphocyte entry. (A) Proliferation rate of PNAd+ and PNAd− endothelial cells (ECs) in wild-type or CD11c-DTR mice that were treated with DT and immunized with OVA/CFA. *, P < 0.01 with the Student's t test compared with PNAd− endothelial cell proliferation in immunized wild-type mice. (B) PNAd+ and PNAd− endothelial cell proliferation plotted as a function of the remaining CD11c+MHCII+ cells in the draining lymph nodes of CD11c-DTR mice. (C) PNAd+ endothelial cell proliferation at day 2 in mice injected with 500,000 BMDCs. BMDCs are unfractionated (All) or enriched for DCs (granulocyte-depleted; Gr-1−). n ≥ 6 mice for each condition. (D) PNAd+ endothelial cell numbers at day 5 after BMDC injection. n = 4. (E) T cell entry into lymph nodes that were stimulated 1 or 8 d before the intravenous transfer of CFSE-labeled splenocytes. Each symbol represents one mouse. Bars represent the mean values. Representative of three separate experiments. *, P < 0.01 with the Student's t test. Error bars represent SD.
Figure 7.
Modulation of VEGF levels in draining lymph nodes. (A and B) VEGF levels in lymph nodes at days 1, 2, and 5 upon injection with either OVA/CFA (A) or 106 granulocyte-depleted BMDCs (B). n = 4 mice for each condition. *, P < 0.02 with the Student's t test compared with control mice injected with PBS. (C) Effect of anti-VEGF on lymph node endothelial cell (EC) proliferation. Mice were treated with PBS (−), control antibody (IgG), or anti-VEGF (αVEGF) and were injected with 106 granulocyte-depleted BMDCs. Endothelial cell BrdU uptake was examined at day 2. n = 4. **, P < 0.002. (D) Effect of DC reduction on lymph node VEGF levels. DT-treated wild-type or CD11c-DTR (DTR) mice were immunized with OVA/CFA and examined on day 1. n = 12 mice for each condition. *, P < 0.05 with the paired Student's t test. (E) Lymph node VEGF levels in RAG1−/− (KO) versus wild-type mice injected with 106 granulocyte-depleted BMDCs and examined on day 1. n = 9. *, P < 0.05 with the Student's t test. (F) Effect of L-selectin blockade on lymph node VEGF levels. Mice were treated with anti–L-selectin (αLsel) or control IgG (IgG), injected with the indicated number of BMDCs, and examined on day 2. n = 6. *, P < 0.02. Error bars represent SD.
Similar articles
- CD11c(hi) dendritic cells regulate the re-establishment of vascular quiescence and stabilization after immune stimulation of lymph nodes.
Tzeng TC, Chyou S, Tian S, Webster B, Carpenter AC, Guaiquil VH, Lu TT. Tzeng TC, et al. J Immunol. 2010 Apr 15;184(8):4247-57. doi: 10.4049/jimmunol.0902914. Epub 2010 Mar 15. J Immunol. 2010. PMID: 20231692 Free PMC article. - Coordinated regulation of lymph node vascular-stromal growth first by CD11c+ cells and then by T and B cells.
Chyou S, Benahmed F, Chen J, Kumar V, Tian S, Lipp M, Lu TT. Chyou S, et al. J Immunol. 2011 Dec 1;187(11):5558-67. doi: 10.4049/jimmunol.1101724. Epub 2011 Oct 26. J Immunol. 2011. PMID: 22031764 Free PMC article. - Multiple CD11c+ cells collaboratively express IL-1β to modulate stromal vascular endothelial growth factor and lymph node vascular-stromal growth.
Benahmed F, Chyou S, Dasoveanu D, Chen J, Kumar V, Iwakura Y, Lu TT. Benahmed F, et al. J Immunol. 2014 May 1;192(9):4153-63. doi: 10.4049/jimmunol.1301765. Epub 2014 Mar 21. J Immunol. 2014. PMID: 24659690 Free PMC article. - Dendritic cell migration through the lymphatic vasculature to lymph nodes.
Platt AM, Randolph GJ. Platt AM, et al. Adv Immunol. 2013;120:51-68. doi: 10.1016/B978-0-12-417028-5.00002-8. Adv Immunol. 2013. PMID: 24070380 Review. - The role of core TNF/LIGHT family members in lymph node homeostasis and remodeling.
Zhu M, Fu YX. Zhu M, et al. Immunol Rev. 2011 Nov;244(1):75-84. doi: 10.1111/j.1600-065X.2011.01061.x. Immunol Rev. 2011. PMID: 22017432 Review.
Cited by
- Tertiary lymphoid structures and B lymphocytes in cancer prognosis and response to immunotherapies.
N J, J T, Sl N, Gt B. N J, et al. Oncoimmunology. 2021 Mar 29;10(1):1900508. doi: 10.1080/2162402X.2021.1900508. Oncoimmunology. 2021. PMID: 33854820 Free PMC article. Review. - Tertiary Lymphoid Structures in Cancers: Prognostic Value, Regulation, and Manipulation for Therapeutic Intervention.
Sautès-Fridman C, Lawand M, Giraldo NA, Kaplon H, Germain C, Fridman WH, Dieu-Nosjean MC. Sautès-Fridman C, et al. Front Immunol. 2016 Oct 3;7:407. doi: 10.3389/fimmu.2016.00407. eCollection 2016. Front Immunol. 2016. PMID: 27752258 Free PMC article. Review. - Optical projection tomography reveals dynamics of HEV growth after immunization with protein plus CFA and features shared with HEVs in acute autoinflammatory lymphadenopathy.
Kumar V, Chyou S, Stein JV, Lu TT. Kumar V, et al. Front Immunol. 2012 Sep 7;3:282. doi: 10.3389/fimmu.2012.00282. eCollection 2012. Front Immunol. 2012. PMID: 22973277 Free PMC article. - The draining lymph node in rheumatoid arthritis: current concepts and research perspectives.
Benaglio F, Vitolo B, Scarabelli M, Binda E, Bugatti S, Caporali R, Montecucco C, Manzo A. Benaglio F, et al. Biomed Res Int. 2015;2015:420251. doi: 10.1155/2015/420251. Epub 2015 Feb 22. Biomed Res Int. 2015. PMID: 25793195 Free PMC article. Review. - TGF-beta1 induces mouse dendritic cells to express VEGF and its receptor (Flt-1) under hypoxic conditions.
Nam EH, Park SR, Kim PH. Nam EH, et al. Exp Mol Med. 2010 Sep 30;42(9):606-13. doi: 10.3858/emm.2010.42.9.059. Exp Mol Med. 2010. PMID: 20631490 Free PMC article.
References
- Ferrara, N., H. Chen, T. Davis-Smyth, H.P. Gerber, T.N. Nguyen, D. Peers, V. Chisholm, K.J. Hillan, and R.H. Schwall. 1998. Vascular endothelial growth factor is essential for corpus luteum angiogenesis. Nat. Med. 4:336–340. - PubMed
- Luttun, A., M. Tjwa, L. Moons, Y. Wu, A. Angelillo-Scherrer, F. Liao, J.A. Nagy, A. Hooper, J. Priller, B. De Klerck, et al. 2002. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat. Med. 8:831–840. - PubMed
- Burwell, R.G. 1962. Studies of the primary and the secondary immune responses of lymph nodes draining homografts of fresh cancellous bone (with particular reference to mechanisms of lymph node reactivity). Ann. NY Acad. Sci. 99:821–860. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- C06 RR012538/RR/NCRR NIH HHS/United States
- T32 AI007621/AI/NIAID NIH HHS/United States
- 5T32 AI007621-07/AI/NIAID NIH HHS/United States
- C06-RR12538-01/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials