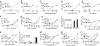Details of Toll-like receptor:adapter interaction revealed by germ-line mutagenesis - PubMed (original) (raw)
. 2006 Jul 18;103(29):10961-6.
doi: 10.1073/pnas.0603804103. Epub 2006 Jul 10.
Philippe Georgel, Chenglong Li, Jungwoo Choe, Karine Crozat, Sophie Rutschmann, Xin Du, Tim Bigby, Suzanne Mudd, Sosathya Sovath, Ian A Wilson, Arthur Olson, Bruce Beutler
Affiliations
- PMID: 16832055
- PMCID: PMC1544157
- DOI: 10.1073/pnas.0603804103
Details of Toll-like receptor:adapter interaction revealed by germ-line mutagenesis
Zhengfan Jiang et al. Proc Natl Acad Sci U S A. 2006.
Abstract
The immunovariant N-ethyl-N-nitrosourea-induced mutations Pococurante (Poc) and Lackadaisical were found to alter MyD88, creating striking receptor-selective effects. Poc, in particular, prevented sensing of all MyD88-dependent Toll-like receptor (TLR) ligands except diacyl lipopeptides. Furthermore, Poc-site and classical BB loop mutations caused equivalent phenotypes when engrafted into any TLR/IL-1 receptor/resistance (TIR) domain. These observations, complemented by data from docking studies and site-directed mutagenesis, revealed that BB loops and Poc sites interact homotypically across the receptor:adapter signaling interface, whereas the C-terminal alpha(E)-helices support adapter:adapter and receptor:receptor oligomerization. We have thus defined the TIR domain surface that mediates association between TLRs and MyD88 and the surface required for MyD88 or TLR oligomerization. Moreover, MyD88 engages individual TLRs differently, suggesting the feasibility of selective pharmacologic TIR domain receptor blockade.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
Fig. 1.
Phenotypes of mutant mice. Peritoneal macrophages from each strain were treated with each specific inducer as indicated. After 4 h of incubation, supernatants were collected and assayed in duplicate for TNF concentrations using the L929 bioassay (Poc in A_–_H, Lkd in J and K, and Int in M_–_O). Values represent mean ± SEM (n = 6 mice or more). (I and L) Macrophages from WT, Poc (I), or Lkd (L) mice were pretreated with IFN-γ (10 units/ml) for 4 h. Cells were washed with medium once and treated with TLR ligands as indicated for another 4 h. The supernatants were collected, and the concentration of type I IFN was assayed by using a L929-ISRE-Luc-based bioassay. Similar results were observed in three independent experiments.
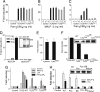
Fig. 2.
Effects of the Poc and Lkd mutations in vivo and ex vivo. (A) The Poc mutation confers sensitivity to MALP-2-induced lethal toxicity. Wild-type, Poc, and MyD88-deficient mice were injected i.p. with 3 μg of MALP-2 and 20 mg of
d
-galactosamine. Survival was monitored over a period of 3 days (no change was observed after 48 h), and the data are expressed as a Kaplan–Meier plot (P < 0.0001). (B and C) The Poc allele of MyD88 supports resistance to skin infection caused by S. pyogenes. Mice were each injected s.c. with S. pyogenes (5 × 105 colony-forming units). During a 5-day period, bacterial growth was monitored daily with the Xenogen IVIS imaging system. (C) Luminescence (photons emitted per second) was measured in a defined and constant region of interest. Data are shown as means ± SEM0 (n ≥ 4 mice). (D and E) Poc mice are hypersusceptible to L. monocytogenes infection. Mice were injected i.v. with L. monocytogenes as described in Materials and Methods. Bioluminescence imaging was performed by using the IVIS Imaging System. The survival of these mice was monitored during a 7-day period. P values refer to comparison with wild type (E). (F and G) Poc mice are hypersusceptible to mouse CMV infection. (F) Viral titers, expressed as log plaque-forming units per spleen, were determined in mice 5 days after i.p. inoculation with 5 × 105 plaque-forming units of mouse CMV. (G) Blood was collected 36 h after infection, and the concentrations of type I IFN in serum were analyzed by ELISA. (H) Macrophages from Poc or _MyD88_−/− mice were treated with either MALP-2 or Pam2CSK4 for the indicated times. Cells were lysed and analyzed by immunoblotting with antibodies indicated. (I) MyD88Poc interacts with TLR2 after MALP-2 treatment. HEK-293 cells were transiently transfected with M2-Flag-tagged TLR2 and HA-tagged WT-MyD88 or MyD88Poc and treated with Pam3CSK4 or MALP-2 for the indicated times. Cells were lysed and immunoprecipitated with M2-Flag followed by immunoblotting with antibody against the HA. The expression of transfected MyD88 and TLR2 was examined in the whole-cell extracts (WCE).
Fig. 3.
The αE-helices of TIR domains are not involved in receptor:adapter interactions. (A_–_C) A total of 100 ng (A) or 10 ng (B and C) of vector control or the indicated TLR constructs were transfected together with 50 ng of pNiFty-luc into HEK-293 cells. After 24 h cells were left untreated or treated with ligands for 4 h and then harvested and assayed for luciferase activity. (D) HEK-293 cells were transfected with HA-tagged MyD88FW/AA and with M2-tagged TLRs. After 36 h, cells were left untreated or treated with MALP2 (for TLR2 transfectants) or lipid A (for TLR4 transfectants) for 10 min. The cells were then lysed and immunoprecipitated with M2-Flag. Immunoblotting was then performed with antibody against the HA tag on MyD88FW/AA. The expression of transfected MyD88FW/AA and TLRs was examined in the whole-cell extracts (WCE). (E and F) Proposed TIR domain interactions based on docking studies. (E) Face-to-face interaction mode mediating receptor:adapter binding. TLR2 is shown in blue, and MyD88 is shown in yellow. Individual amino acids and motifs are indicated by arrows in colors corresponding to the color of each protein. The critical P residue of each BB loop is shown in red, and the critical V or I residue of the Poc site is shown in green. (F) Back-to-back interaction mode mediating TIR domain oligomerization. Blue and yellow ribbons may now be taken to represent TIR domains of two different MyD88 proteins or two different TLR2 molecules after ligand stimulation. The interaction is mediated by the C-terminal α-helices (αE) in an antiparallel fashion.
Fig. 4.
Diacylated lipopeptides activate TLR2 in a unique way. (A_–_C) Ten nanograms of vector control (pCMV-Flag), TLR2-WT, or TLR2Poc was transfected together with 50 ng of pNiFty-luc into HEK-293 cells. After 24 h, cells were left untreated or treated with TLR2 ligands at different concentrations as indicated. Four hours later, luciferase reporter assay was performed. (D) HEK-293 cells were transfected with 50 ng of pNiFty-luc construct together with indicated amount of MD-2 (0, 20, and 50 ng) and TLR4-WT or TLR4Poc (0, 20, and 50 ng). After 24 h, cells were harvested and analyzed by luciferase reporter assay. Cell extracts were also used to examine the expression of TLR4 and MD2 by immunoblotting (Inset). (E and F) HEK-293 cells were transfected with 50 ng of pNiFty-luc and 50 ng of each construct as indicated. A luciferase reporter assay was performed after 24 h. Cell extracts were used to examine the expression of the wild-type and mutant TLR2 variants by using antibody against M2-Flag (Inset). (G and H) MEFs derived from _Tlr2_−/− (G) or _MyD88_−/− (H) mice were transfected with 20 ng of pNiFty-luc and 20 ng of different TLR2 or MyD88 expression vectors as indicated. Twenty-four hours after transfection, cells were left untreated or treated with TLR2 ligands as indicated for 6 h and analyzed with luciferase assay. Cell extracts were also used to examine the expression of the wild-type and mutant MyD88 by using antibody against the HA tag (Inset).
Similar articles
- Signalling of toll-like receptors.
Brikos C, O'Neill LA. Brikos C, et al. Handb Exp Pharmacol. 2008;(183):21-50. doi: 10.1007/978-3-540-72167-3_2. Handb Exp Pharmacol. 2008. PMID: 18071653 Review. - Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6.
Häcker H, Redecke V, Blagoev B, Kratchmarova I, Hsu LC, Wang GG, Kamps MP, Raz E, Wagner H, Häcker G, Mann M, Karin M. Häcker H, et al. Nature. 2006 Jan 12;439(7073):204-7. doi: 10.1038/nature04369. Epub 2005 Nov 23. Nature. 2006. PMID: 16306937 - Simultaneous deletion of MyD88 and Trif delays major histocompatibility and minor antigen mismatch allograft rejection.
McKay D, Shigeoka A, Rubinstein M, Surh C, Sprent J. McKay D, et al. Eur J Immunol. 2006 Aug;36(8):1994-2002. doi: 10.1002/eji.200636249. Eur J Immunol. 2006. PMID: 16874736 - Distinct roles of TIR and non-TIR regions in the subcellular localization and signaling properties of MyD88.
Nishiya T, Kajita E, Horinouchi T, Nishimoto A, Miwa S. Nishiya T, et al. FEBS Lett. 2007 Jul 10;581(17):3223-9. doi: 10.1016/j.febslet.2007.06.008. Epub 2007 Jun 15. FEBS Lett. 2007. PMID: 17583698 - MAPPIT analysis of early Toll-like receptor signalling events.
Ulrichts P, Tavernier J. Ulrichts P, et al. Immunol Lett. 2008 Mar 15;116(2):141-8. doi: 10.1016/j.imlet.2007.11.026. Epub 2008 Jan 7. Immunol Lett. 2008. PMID: 18221795 Review.
Cited by
- The Tpl2 mutation Sluggish impairs type I IFN production and increases susceptibility to group B streptococcal disease.
Xiao N, Eidenschenk C, Krebs P, Brandl K, Blasius AL, Xia Y, Khovananth K, Smart NG, Beutler B. Xiao N, et al. J Immunol. 2009 Dec 15;183(12):7975-83. doi: 10.4049/jimmunol.0902718. J Immunol. 2009. PMID: 19923465 Free PMC article. - Blocking TIR Domain Interactions in TLR9 Signaling.
Javmen A, Szmacinski H, Lakowicz JR, Toshchakov VY. Javmen A, et al. J Immunol. 2018 Aug 1;201(3):995-1006. doi: 10.4049/jimmunol.1800194. Epub 2018 Jun 18. J Immunol. 2018. PMID: 29914886 Free PMC article. - Chemoproteomics reveals Toll-like receptor fatty acylation.
Chesarino NM, Hach JC, Chen JL, Zaro BW, Rajaram MV, Turner J, Schlesinger LS, Pratt MR, Hang HC, Yount JS. Chesarino NM, et al. BMC Biol. 2014 Nov 5;12:91. doi: 10.1186/s12915-014-0091-3. BMC Biol. 2014. PMID: 25371237 Free PMC article. - Enhanced Mucosal Defense and Reduced Tumor Burden in Mice with the Compromised Negative Regulator IRAK-M.
Rothschild DE, Zhang Y, Diao N, Lee CK, Chen K, Caswell CC, Slade DJ, Helm RF, LeRoith T, Li L, Allen IC. Rothschild DE, et al. EBioMedicine. 2017 Feb;15:36-47. doi: 10.1016/j.ebiom.2016.11.039. Epub 2016 Dec 3. EBioMedicine. 2017. PMID: 27939424 Free PMC article. - Structural and Functional Attributes of the Interleukin-36 Receptor.
Yi G, Ybe JA, Saha SS, Caviness G, Raymond E, Ganesan R, Mbow ML, Kao CC. Yi G, et al. J Biol Chem. 2016 Aug 5;291(32):16597-609. doi: 10.1074/jbc.M116.723064. Epub 2016 Jun 15. J Biol Chem. 2016. PMID: 27307043 Free PMC article.
References
- Beutler B., Hoebe K., Du X., Ulevitch R. J. J. Leukocyte Biol. 2003;74:479–485. - PubMed
- Takeda K., Akira S. Genes Cells. 2001;6:733–742. - PubMed
- Akira S., Takeda K., Kaisho T. Nat. Immunol. 2001;2:675–680. - PubMed
- Kawai T., Adachi O., Ogawa T., Takeda K., Akira S. Immunity. 1999;11:115–122. - PubMed
- Fitzgerald K. A., Palsson-McDermott E. M., Bowie A. G., Jefferies C. A., Mansell A. S., Brady G., Brint E., Dunne A., Gray P., Harte M. T., et al. Nature. 2001;413:78–83. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials