Comparison of lentiviral vector titration methods - PubMed (original) (raw)
Comparative Study
Comparison of lentiviral vector titration methods
Martine Geraerts et al. BMC Biotechnol. 2006.
Abstract
Background: Lentiviral vectors are efficient vehicles for stable gene transfer in dividing and non-dividing cells. Several improvements in vector design to increase biosafety and transgene expression, have led to the approval of these vectors for use in clinical studies. Methods are required to analyze the quality of lentiviral vector production, the efficiency of gene transfer and the extent of therapeutic gene expression.
Results: We compared lentiviral vector titration methods that measure pg p24/ml, RNA equivalents/ml, transducing units (TU/ml) or mRNA equivalents. The amount of genomic RNA in vector particles proves to be reliable to assess the production quality of vectors encoding non-fluorescent proteins. However, the RNA and p24 titers of concentrated vectors are rather poor in predicting transduction efficiency, due to the high variability of vector production based on transient transfection. Moreover, we demonstrate that transgenic mRNA levels correlate well with TU and can be used for functional titration of non-fluorescent transgenes.
Conclusion: The different titration methods have specific advantages and disadvantages. Depending on the experimental set-up one titration method should be preferred over the others.
Figures
Figure 1
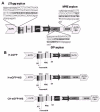
Overview of primer sets and lentiviral vector constructs. (A) Schematic representation of CH-eGFP-WS lentiviral vector with the corresponding amplicons of the different primer sets (LTR-gag, GFP and WPRE). Primer sequences are represented in small caps and probe sequences are in bold. (B) Schematic representation of different lentiviral vector constructs. The construct was optimized to increase transduction efficiency (cPPT and WPRE) and biosafety (SIN) as described before [9, 10, 23].
Figure 2
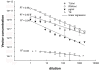
Comparison of lentiviral vector titration methods. CH-eGFP-WS vector was serially diluted (1/2) and subjected to RT-qPCR, ELISA and FACS analysis after transduction of 293T cells, to determine the linearity of the different titration methods. The correlation coefficients are representative for 3 independent experiments. The asterix (*) represents values that were not included in the linear regression.
Figure 3
Kinetics of lentiviral vector production. The CH-eGFP-WS-derived lentiviral vector was produced in two CF2 modules by triple transient transfection in serum-free medium. Starting at 2 days post-transfection, cell-culture medium was harvested once a day for five consecutive days and concentrated by low-speed centrifugation. The p24 content (pg p24/ml) of vector preparations was determined by ELISA, the transducing titers (TU/ml) by FACS analysis and the RNA equivalents (RNA/ml) by one-step RT-qPCR with LTR primers. Data represent the mean value ± standard deviation and represent 3 independent experiments. A decrease in vector titer starting at 3 days post-transfection was evidenced by all methods. The specific activities (TU/pg and TU/RNA) are depicted in the inset.
Figure 4
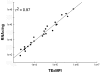
Correlation of eGFP fluorescence and mRNA transgene expression levels. Four independently produced preparations of CH-eGFP-WS-derived lentiviral vector were serially diluted (1/10) prior to transduction of 293T cells. 6 days later, cells were harvested for eGFP analysis by FACS or RNA extraction and subsequent RT-qPCR with primers and probe directed against WPRE to measure transgene expression and against RNAse P to normalize for total RNA content. Total eGFP expression was measured by multiplying the percentage of transduced cells (TE) with the mean fluorescence intensity (MFI). The transgene mRNA level is given as the number of mRNA copies normalized to the total RNA content (RNAc/ng). These values correlate strongly with each other (r2 = 0.97).
Similar articles
- Titering lentiviral vectors: comparison of DNA, RNA and marker expression methods.
Sastry L, Johnson T, Hobson MJ, Smucker B, Cornetta K. Sastry L, et al. Gene Ther. 2002 Sep;9(17):1155-62. doi: 10.1038/sj.gt.3301731. Gene Ther. 2002. PMID: 12170379 - Production of lentiviral vectors for transducing cells from the central nervous system.
Li M, Husic N, Lin Y, Snider BJ. Li M, et al. J Vis Exp. 2012 May 24;(63):e4031. doi: 10.3791/4031. J Vis Exp. 2012. PMID: 22664962 Free PMC article. - Quantitative polymerase chain reaction as a reliable method to determine functional lentiviral titer after ex vivo gene transfer in human mesenchymal stem cells.
Böcker W, Rossmann O, Docheva D, Malterer G, Mutschler W, Schieker M. Böcker W, et al. J Gene Med. 2007 Jul;9(7):585-95. doi: 10.1002/jgm.1049. J Gene Med. 2007. PMID: 17510916 - Real-time quantitative PCR for the design of lentiviral vector analytical assays.
Delenda C, Gaillard C. Delenda C, et al. Gene Ther. 2005 Oct;12 Suppl 1:S36-50. doi: 10.1038/sj.gt.3302614. Gene Ther. 2005. PMID: 16231054 Review. - Lentiviral vectors: are they the future of animal transgenesis?
Park F. Park F. Physiol Genomics. 2007 Oct 22;31(2):159-73. doi: 10.1152/physiolgenomics.00069.2007. Epub 2007 Aug 7. Physiol Genomics. 2007. PMID: 17684037 Review.
Cited by
- DNA damage contributes to age-associated differences in SARS-CoV-2 infection.
Jin R, Niu C, Wu F, Zhou S, Han T, Zhang Z, Li E, Zhang X, Xu S, Wang J, Tian S, Chen W, Ye Q, Cao C, Cheng L. Jin R, et al. Aging Cell. 2022 Dec;21(12):e13729. doi: 10.1111/acel.13729. Epub 2022 Oct 18. Aging Cell. 2022. PMID: 36254583 Free PMC article. - Boosting antitumor response with PSMA-targeted immunomodulatory VLPs, harboring costimulatory TNFSF ligands and GM-CSF cytokine.
Palameta S, Manrique-Rincón AJ, Toscaro JM, Semionatto IF, Fonseca MC, Rosa RSM, Ruas LP, Oliveira PSL, Bajgelman MC. Palameta S, et al. Mol Ther Oncolytics. 2022 Feb 17;24:650-662. doi: 10.1016/j.omto.2022.02.010. eCollection 2022 Mar 17. Mol Ther Oncolytics. 2022. PMID: 35284623 Free PMC article. - Paired Related Homeobox Protein 1 Regulates Quiescence in Human Oligodendrocyte Progenitors.
Wang J, Saraswat D, Sinha AK, Polanco J, Dietz K, O'Bara MA, Pol SU, Shayya HJ, Sim FJ. Wang J, et al. Cell Rep. 2018 Dec 18;25(12):3435-3450.e6. doi: 10.1016/j.celrep.2018.11.068. Cell Rep. 2018. PMID: 30566868 Free PMC article. - The Functional Consequences of the Novel Ribosomal Pausing Site in SARS-CoV-2 Spike Glycoprotein RNA.
Postnikova OA, Uppal S, Huang W, Kane MA, Villasmil R, Rogozin IB, Poliakov E, Redmond TM. Postnikova OA, et al. Int J Mol Sci. 2021 Jun 17;22(12):6490. doi: 10.3390/ijms22126490. Int J Mol Sci. 2021. PMID: 34204305 Free PMC article. - Vaccines delivered by integration-deficient lentiviral vectors targeting dendritic cells induces strong antigen-specific immunity.
Hu B, Dai B, Wang P. Hu B, et al. Vaccine. 2010 Sep 24;28(41):6675-83. doi: 10.1016/j.vaccine.2010.08.012. Epub 2010 Aug 13. Vaccine. 2010. PMID: 20709004 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources