Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis - PubMed (original) (raw)
doi: 10.1016/j.ccr.2006.06.001.
Robin Mathew, Brian Beaudoin, Kevin Bray, Diana Anderson, Guanghua Chen, Chandreyee Mukherjee, Yufang Shi, Céline Gélinas, Yongjun Fan, Deirdre A Nelson, Shengkan Jin, Eileen White
Affiliations
- PMID: 16843265
- PMCID: PMC2857533
- DOI: 10.1016/j.ccr.2006.06.001
Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis
Kurt Degenhardt et al. Cancer Cell. 2006 Jul.
Abstract
Defective apoptosis renders immortalized epithelial cells highly tumorigenic, but how this is impacted by other common tumor mutations is not known. In apoptosis-defective cells, inhibition of autophagy by AKT activation or by allelic disruption of beclin1 confers sensitivity to metabolic stress by inhibiting an autophagy-dependent survival pathway. While autophagy acts to buffer metabolic stress, the combined impairment of apoptosis and autophagy promotes necrotic cell death in vitro and in vivo. Thus, inhibiting autophagy under conditions of nutrient limitation can restore cell death to apoptosis-refractory tumors, but this necrosis is associated with inflammation and accelerated tumor growth. Thus, autophagy may function in tumor suppression by mitigating metabolic stress and, in concert with apoptosis, by preventing death by necrosis.
Figures
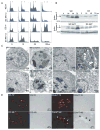
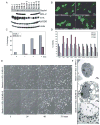
Figure 1. AKT and RAS promote tumor growth in both wild-type and apoptosis-defective genetic backgrounds
A: Western blots showing protein expression in the W2 and D3 derived vector control and AKT-expressing iBMK cell lines. B: Tumor growth kinetics of iBMK cell lines. W2 vector controls (blue) compared to W2 derivatives expressing RAS (yellow). W2 vector control (blue) compared to W2 derivatives expressing _myr-_AKT (green). BAX/BAK-deficient D3 (red) and W2 plus RAS (yellow) are included for comparison. Addition of RAS to BAX/BAK-deficient D3 cells accelerates tumor growth as depicted by D3 (red) and D3 plus RAS (yellow). AKT promotes tumor growth of apoptosis-defective D3 cells as depicted by D3 AKT cells (purple). W2 (blue), W2 plus AKT (green), D3 and D3 vector controls (red), and W2 plus RAS (yellow) are shown for comparison. C: H&E staining of W2, W2 AKT, D3, and D3 AKT tumors illustrating tumor giant cells (TGC), necrosis (N), necrotic cells (NC), blood vessels (BV), and healthy tumor (TUMOR). Boxes indicate location of magnified areas. D: Hypoxyprobe staining (Nelson et al., 2004) of D3 AKT and D3 tumors indicating hypoxic conditions associated with necrotic regions in D3 AKT tumors. E: H&E staining of W2 BCL-2 tumors and W2 BCL-2 AKT tumors (Nelson et al., 2004) illustrating the pronounced necrosis (N) induced by AKT activation independent of the means of apoptosis inhibition.
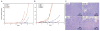
Figure 2. Ischemia induces autophagy in BAX/BAK-deficient iBMK cells that is diverted to necrosis by AKT activation
A: Flow cytometry of W2, W2 AKT, D3, and D3 AKT cells treated with ischemia in vitro for the indicated number of hours. Arrows indicate the positions of 2N/G1 (left) and 4N/G2/M (right) DNA content. B: Induction of caspase-3 activation in W2 and W2 AKT but not in D3 or D3 AKT cells in response to ischemia in vitro. Western blot of cell extracts indicates p17 active caspase-3 band (arrows) and nonspecific band (*). C: EM of untreated and ischemic (48 hr) W2, W2 AKT, D3, and D3 AKT cells from A. Note the apoptotic morphology of W2 and W2 AKT cells, the autophagy morphology of D3 cells (arrows indicate membranous organelle-like accumulations within vacuoles), and the necrotic morphology with vacuolated cytoplasm of D3 AKT cells. Insets represent progressive and distinctive endpoints indicative of autophagy (D3), or necrosis displaying a completely condensed nucleus (D3 AKT). D: Loss of nuclear HMGB1 staining in necrotic D3 AKT (arrows) but not D3 cells in ischemia (48 hr) as a marker of necrosis.
Figure 3. Ischemia induces autophagy in apoptosis-defective cells that when inhibited by AKT promotes cell death by necrosis
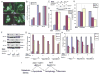
A: BAX/BAK-deficient D3 cells without and with AKT expressing the autophagy marker EGFP-LC3 were untreated or placed under ischemic conditions for 24 hr. EGFP-LC3 displayed diffuse intracellular localization under normal growth conditions, and membrane translocation (punctate localization) indicative of autophagy was observed in ischemic D3 cells, which was substantially inhibited in D3 AKT cells. B: Quantitation of EGFP-LC3 translocation in untreated and ischemic D3 and D3 AKT cells (representative of three independent experiments). Note that, although the D3 AKT cells treated with ischemia have a high proportion of cells with translocated LC3 by 2 days in ischemia, viability is low. C: Western blot showing knockdown of Beclin1 in D3 cells relative to the LaminA/C control at 24, 48, and 72 hr posttransfection under normal culture conditions; hours posttransfection correspond to 0, 24, and 48 hr of ischemia in D and E. D: Reduction of autophagy in D3 cells by Beclin1 RNAi from C assessed by EGFP-LC3 translocation. E: Knockdown of Beclin1 impairs survival of D3 cells to metabolic stress. Viability of D3 cells from C and D was assessed at 0, 24, and 48 hr of ischemia. F: Western blot time course of Beclin1 knockdown by RNAi relative to the LaminA/C control under normal culture conditions in HeLa vector control cells and HeLa cells expressing BCL-xL or BCL-2. G: Knockdown of Beclin1 in HeLa cells impairs autophagy induction by ischemia. Quantitation of EGFP-LC3 translocation in untreated (0 hr) and ischemic treated (24 and 48 hr) HeLa vector controls or HeLa cells expressing BCL-xL or BCL-2 following Beclin1 or LaminA/C knockdown for 24 hr by RNAi. H: Knockdown of Beclin1 in HeLa cells expressing BCL-xL or BCL-2 under ischemic conditions induces cell death. Viability of HeLa cells without or with BCL-xL or BCL-2 in response to ischemia with or without knockdown of Beclin1 by RNAi is described in E and F. I: Model for functional ordering and regulation of death pathways leading to necrosis. Values represent the mean ± standard deviation for n ≥ 3. Error bars represent ± one standard deviation.
Figure 4. Distinct morphological features of apoptosis, necrosis, and autophagy in ischemia
A: Identical frames from 5 day time-lapse videos of the indicated cell lines in ischemia (100×). No morphological changes were apparent in W2 or D3AKT cells beyond 72 and 48 hr, respectively (data not shown). Boxed cells and inset (enlarged) show D3 AKT cells undergoing lysis. At 112 hr of ischemia, D3 cells were returned to normal culture conditions and photographed 120 hr later to document recovery. A representative frame of the recovery of D3 cells is shown. B: Representative individual cells from A were followed for the indicated times beginning immediately prior to any signs of altered morphology in ischemia beginning at 13.5 hr for W2, 16.6 hr for D3 AKT, and 52.6 hr for D3. C: Impact of different modes of cell death on clonogenic survival. W2, W2 AKT, D3, and D3 AKT cells were treated with ischemia for 3, 5, and 7 days, and the potential for clonogenic growth was determined following return to normal growth conditions (one of two replicates).
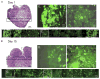
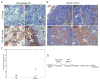
Figure 5. Autophagy in tumors localizes to regions of metabolic stress prior to angiogenesis
Tumors were established from D3 cells stably expressing EGFP-LC3 and monitored for membrane translocation indicative of autophagy at days 1 (A) and 15 (B) following implantation. Following excision, tumors were processed for histology (frozen sections) to preserve EGFP-LC3 fluorescence (Mizushima et al., 2004); a section through the entire tumor at day 1 and 1/4 of the tumor at day 15 is shown by H&E (magnification 40×), and a panorama across the tumor is shown for fluorescence (magnification 600×), as indicated. “E” and “C” indicate individual images from the tumor edge and center, respectively, enlarged to highlight the differential localization of EGFP-LC3 at day 1 that is diffuse at the tumor periphery and punctate in the center. Due to the compactness of the cells in the tumor, punctations may represent more than one autophagosome. Day 15 tumors have recruited a blood supply and display diffuse EGFP-LC3 localization throughout the tumor.
Figure 6. beclin1 haploinsufficiency impairs survival in ischemia in an apoptosis-defective background
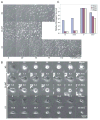
A: Western blot analysis of Beclin1, BCL-2, E1A, p53DD, and actin in beclin1+/+ and beclin1+/− iBMK cell lines with and without BCL-2 expression. B: beclin1 haploinsufficiency impairs autophagy. beclin1+/+ and beclin1+/− iBMK cells expressing BCL-2 (WB-3 and BLNB-12) were transfected with the EGFP-LC3 expression vector and at 24 hr posttransfection were subjected to 0 and 24 hr of ischemia and were monitored for membrane translocation by fluorescence microscopy. Representative fields are shown. C: Quantitation of EGFP-LC3 translocation (% autophagy) in WB-3 and BLNB-12 in B following 0, 1, 2, and 3 days in ischemia. D: beclin1 haploinsufficiency impairs viability in ischemia only when apoptosis is inhibited. beclin1+/+ and beclin1+/− iBMKs with and without BCL-2 were incubated in ischemic conditions, and viability was assessed by trypan blue staining at the indicated times. E: Time course of morphological alterations due to beclin1 haploinsufficiency in iBMK cells expressing BCL-2 (WB-13 and BLNB-12). beclin1+/+ cells expressing BCL-2 proliferate, followed by cellular condensation, whereas beclin1+/− cells expressing BCL-2 display diminished capacity to sustain proliferation and show accelerated necrosis. F: EM (5000×) of beclin1+/+ BCL-2 (WB-13) and beclin1+/− BCL-2 (BLNB-12) following 7 days in ischemia. Note the condensed morphology of WB-13 and the vacuolated and necrotic morphology of BLNB-12.
Figure 7. beclin1 haploinsufficiency promotes epithelial tumorigenesis
A: Tumor growth kinetics of two beclin1+/+ (W4-2, W4-1) and two beclin1+/− (BCLN34-3, BCLN4-1) iBMK cell lines. B: Tumor growth kinetics of two beclin1+/+, BCL-2-expressing (WB-10, WB-13) and two beclin1+/−, BCL-2-expressing (BLNB-4, BCLNB-13) iBMK cell lines. C: Histology of two beclin1+/+, BCL-2 (left) and two beclin1+/−, BCL-2 (right) tumors. One out of eight beclin1+/+, BCL-2 tumors and six out of eight beclin1+/−, BCL-2 tumors showed evidence of necrosis (N). All tumors were between 500 and 1000 mm3 at the time of histological analysis.
Figure 8. Tumor necrosis is associated with macrophage infiltration and cytokine and chemokine production
A: The macrophage marker Mac3 staining of D3 (B4) and D3 AKT (D5) tumors by IHC with brown staining indicating reactivity in necrotic areas (100×, insets 400×). Note the high level of reactivity in necrotic areas of D3 AKT tumors and the near absence of staining in D3 tumors. B: IHC for p50 NF-κB as in A demonstrating enhanced staining in D3 AKT tumors. C: In vivo tumor necrosis is associated with enhanced activity of the cytokine-responsive IL6 promoter. Tumors were generated subcutaneously by injecting D3 or D3AKT cells stably expressing a luciferase reporter gene under the control of three NF-κB DNA binding motifs from the IL6 promoter and monitored for in vivo promoter activation. To compare the relative luminescence signals in D3 and D3 AKT tumors, the total photon values were normalized to basal luminescence activity at day 2 postinjection and compared to that after tumor growth where tumor sizes were comparable: day 15 for D3 AKT with an average tumor volume of 88.9 mm3, day 29 for D3 with an average tumor volume of 82.2 mm3. The graph represents relative IL6-LUC activity from five individual tumors of each genotype with the mean indicated by the bar. D: Model for hierarchy of death pathways and impact on tumor growth.
Similar articles
- Metabolic catastrophe as a means to cancer cell death.
Jin S, DiPaola RS, Mathew R, White E. Jin S, et al. J Cell Sci. 2007 Feb 1;120(Pt 3):379-83. doi: 10.1242/jcs.03349. J Cell Sci. 2007. PMID: 17251378 Free PMC article. Review. - Autophagy suppresses tumor progression by limiting chromosomal instability.
Mathew R, Kongara S, Beaudoin B, Karp CM, Bray K, Degenhardt K, Chen G, Jin S, White E. Mathew R, et al. Genes Dev. 2007 Jun 1;21(11):1367-81. doi: 10.1101/gad.1545107. Epub 2007 May 17. Genes Dev. 2007. PMID: 17510285 Free PMC article. - Role of autophagy in cancer: management of metabolic stress.
Jin S, White E. Jin S, et al. Autophagy. 2007 Jan-Feb;3(1):28-31. doi: 10.4161/auto.3269. Epub 2007 Jan 3. Autophagy. 2007. PMID: 16969128 Free PMC article. Review. - Bif-1 interacts with Beclin 1 through UVRAG and regulates autophagy and tumorigenesis.
Takahashi Y, Coppola D, Matsushita N, Cualing HD, Sun M, Sato Y, Liang C, Jung JU, Cheng JQ, Mulé JJ, Pledger WJ, Wang HG. Takahashi Y, et al. Nat Cell Biol. 2007 Oct;9(10):1142-51. doi: 10.1038/ncb1634. Epub 2007 Sep 23. Nat Cell Biol. 2007. PMID: 17891140 Free PMC article. - Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis.
Karantza-Wadsworth V, Patel S, Kravchuk O, Chen G, Mathew R, Jin S, White E. Karantza-Wadsworth V, et al. Genes Dev. 2007 Jul 1;21(13):1621-35. doi: 10.1101/gad.1565707. Genes Dev. 2007. PMID: 17606641 Free PMC article.
Cited by
- Anti-Cancer Strategy Based on Changes in the Role of Autophagy Depending on the Survival Environment and Tumorigenesis Stages.
Lee M, Kim HG. Lee M, et al. Molecules. 2024 Oct 30;29(21):5134. doi: 10.3390/molecules29215134. Molecules. 2024. PMID: 39519774 Free PMC article. Review. - Beclin-1 and LC3B expression in canine mast cell tumours: an immuno-ultrastructural and immunohistochemical study of autophagy.
Vicente GP, Della Salda L, Strefezzi RF. Vicente GP, et al. Vet Q. 2024 Dec;44(1):1-15. doi: 10.1080/01652176.2024.2419585. Epub 2024 Nov 1. Vet Q. 2024. PMID: 39483060 Free PMC article. - Mechanisms of drug resistance in nutrient-depleted colorectal cancer cells: insights into lysosomal and mitochondrial drug sequestration.
Köse SG, Güleç Taşkıran AE. Köse SG, et al. Biol Open. 2024 Jul 15;13(10):bio060448. doi: 10.1242/bio.060448. Epub 2024 Oct 24. Biol Open. 2024. PMID: 39445740 Free PMC article. Review. - Promising and challenging phytochemicals targeting LC3 mediated autophagy signaling in cancer therapy.
Rajendran P, Renu K, Ali EM, Genena MAM, Veeraraghavan V, Sekar R, Sekar AK, Tejavat S, Barik P, Abdallah BM. Rajendran P, et al. Immun Inflamm Dis. 2024 Oct;12(10):e70041. doi: 10.1002/iid3.70041. Immun Inflamm Dis. 2024. PMID: 39436197 Free PMC article. Review. - Autophagy is required for mammary tumor recurrence by promoting dormant tumor cell survival following therapy.
Dwyer S, Ruth J, Seidel HE, Raz AA, Chodosh LA. Dwyer S, et al. Breast Cancer Res. 2024 Oct 18;26(1):143. doi: 10.1186/s13058-024-01878-7. Breast Cancer Res. 2024. PMID: 39425240 Free PMC article.
References
- Adams JM. Ways of dying: multiple pathways to apoptosis. Genes Dev. 2003;17:2481–2495. - PubMed
- Arico S, Petiot A, Bauvy C, Dubbelhuis PF, Meijer AJ, Codogno P, Ogier-Denis E. The tumor suppressor PTEN positively regulates macroautophagy by inhibiting the phosphatidylinositol 3-kinase/protein kinase B pathway. J Biol Chem. 2001;276:35243–35246. - PubMed
- Balkwill F, Coussens LM. Cancer: An inflammatory link. Nature. 2004;431:405–406. - PubMed
- Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–217. - PubMed
- Danial NN, Korsmeyer SJ. Cell death: Critical control points. Cell. 2004;116:205–219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 CA053370/CA/NCI NIH HHS/United States
- R37 CA053370-15/CA/NCI NIH HHS/United States
- R37 CA053370-16/CA/NCI NIH HHS/United States
- R37 CA53370/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials