Pathogenesis of Helicobacter pylori infection - PubMed (original) (raw)
Review
Pathogenesis of Helicobacter pylori infection
Johannes G Kusters et al. Clin Microbiol Rev. 2006 Jul.
Abstract
Helicobacter pylori is the first formally recognized bacterial carcinogen and is one of the most successful human pathogens, as over half of the world's population is colonized with this gram-negative bacterium. Unless treated, colonization usually persists lifelong. H. pylori infection represents a key factor in the etiology of various gastrointestinal diseases, ranging from chronic active gastritis without clinical symptoms to peptic ulceration, gastric adenocarcinoma, and gastric mucosa-associated lymphoid tissue lymphoma. Disease outcome is the result of the complex interplay between the host and the bacterium. Host immune gene polymorphisms and gastric acid secretion largely determine the bacterium's ability to colonize a specific gastric niche. Bacterial virulence factors such as the cytotoxin-associated gene pathogenicity island-encoded protein CagA and the vacuolating cytotoxin VacA aid in this colonization of the gastric mucosa and subsequently seem to modulate the host's immune system. This review focuses on the microbiological, clinical, immunological, and biochemical aspects of the pathogenesis of H. pylori.
Figures
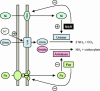
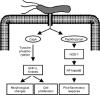
FIG. 1.
Schematic representation of the relationships between acid resistance (urease activity and urea transport), nitrogen metabolism (ammonia production), metal metabolism (iron uptake and nickel uptake), and gene regulation (Fur and NikR) in H. pylori.
FIG. 2.
Schematic representation of the factors contributing to gastric pathology and disease outcome in H. pylori infection.
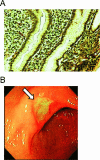
FIG. 3.
(A) Gastric glands abundantly colonized with Helicobacter pylori, shown as dark, curved bacilli closely aligning with the mucosal surface. (B) Endoscopic view of a gastric ulcer, with a clean base at the angulus.
FIG. 4.
Acid secretion and the associated pattern of gastritis play an important role in disease outcome in H. pylori infection. The figure displays the correlations between the pattern of H. pylori colonization, inflammation, acid secretion, gastric and duodenal histology, and clinical outcome.
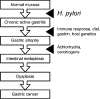
FIG. 5.
Model representing the role of H. pylori and other factors in gastric carcinogenesis, based on the cascade proposed by Correa et al. (96).
FIG. 6.
Schematic representation of the different roles of the Cag type IV secretion system in immune modulation, cell proliferation, and morphological changes.
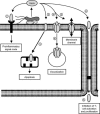
FIG. 7.
The VacA protein influences cellular processes via different routes, thus assisting in chronic colonization of the gastric mucosa by H. pylori. (1) Surface-bound VacA may be directly delivered to the cell membrane. Secreted VacA may either (2) bind to a cell membrane receptor and initiate a proinflammatory response, (3) be taken up directly by the cell and be trafficked to the mitochondria and induce apoptosis, (4) be taken up by pinocytosis and induce vacuolization, (5) form a membrane channel, resulting in leakage of nutrients to the extracellular space, or (6) pass through the tight junctions and inhibit T-cell activation and proliferation. (Modified with permission from Nature Reviews Microbiology [101], copyright Macmillan Magazines Ltd.)
References
- Achtman, M., and S. Suerbaum. 2000. Sequence variation in Helicobacter pylori. Trends Microbiol. 8:57-58. - PubMed
- Akada, J. K., M. Shirai, H. Takeuchi, M. Tsuda, and T. Nakazawa. 2000. Identification of the urease operon in Helicobacter pylori and its control by mRNA decay in response to pH. Mol. Microbiol. 36:1071-1084. - PubMed
- Akhiani, A. A., J. Pappo, Z. Kabok, K. Schon, W. Gao, L. E. Franzen, and N. Lycke. 2002. Protection against Helicobacter pylori infection following immunization is IL-12-dependent and mediated by Th1 cells. J. Immunol. 169:6977-6984. - PubMed
- Akin, O. Y., V. M. Tsou, and A. L. Werner. 1995. Gastrospirillum hominis-associated chronic active gastritis. Pediatr. Pathol. Lab. Med. 15:429-435. - PubMed
- Akopyants, N. S., S. W. Clifton, D. Kersulyte, J. E. Crabtree, B. E. Youree, C. A. Reece, N. O. Bukanov, E. S. Drazek, B. A. Roe, and D. E. Berg. 1998. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol. Microbiol. 28:37-53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical