Transgenic mice rich in endogenous omega-3 fatty acids are protected from colitis - PubMed (original) (raw)
Transgenic mice rich in endogenous omega-3 fatty acids are protected from colitis
Christian A Hudert et al. Proc Natl Acad Sci U S A. 2006.
Abstract
Omega-6 (n-6) and omega-3 (n-3) polyunsaturated fatty acids (PUFA) are the precursors of potent lipid mediators and play an important role in regulation of inflammation. Generally, n-6 PUFA promote inflammation whereas n-3 PUFA have antiinflammatory properties, traditionally attributed to their ability to inhibit the formation of n-6 PUFA-derived proinflammatory eicosanoids. Newly discovered resolvins and protectins are potent antiinflammatory lipid mediators derived directly from n-3 PUFA with distinct pathways of action. However, the role of the n-3 PUFA tissue status in the formation of these antiinflammatory mediators has not been addressed. Here we show that an increased n-3 PUFA tissue status in transgenic mice that endogenously biosynthesize n-3 PUFA from n-6 PUFA leads to significant formation of antiinflammatory resolvins and effective reduction in inflammation and tissue injury in colitis. The endogenous increase in n-3 PUFA and related products did not decrease n-6 PUFA-derived lipid mediators such as leukotriene B4 and prostaglandin E2. The observed inflammation protection might result from decreased NF-kappaB activity and expression of TNFalpha, inducible NO synthase, and IL-1beta, with enhanced mucoprotection probably because of the higher expression of trefoil factor 3, Toll-interacting protein, and zonula occludens-1. These results thus establish the fat-1 transgenic mouse as a new experimental model for the study of n-3 PUFA-derived lipid mediators. They add insight into the molecular mechanisms of inflammation protection afforded by n-3 PUFA through formation of resolvins and protectins other than inhibition of n-6 PUFA-derived eicosanoid formation.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
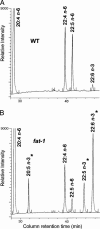
Fig. 1.
Differential fatty acid profiles in WT and fat-1 transgenic mice. Whereas high levels of n-6 fatty acids characterize WT samples (A), n-3 fatty acids are nearly absent. In contrast, an abundance of EPA (20:5 n-3), docosapentaenoic acid (22:5 n-3), and DHA (22:6 n-3) can be found in fat-1 transgenic mice (B). The n-3 PUFA are marked with asterisks.
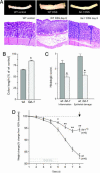
Fig. 2.
Colon inflammation activity in WT and fat-1 transgenic mice. (A) Macroscopic view (Upper) and microscopic hematoxylin and eosin staining (Lower) of the distal colon in WT control mice (Left), DSS-treated WT nontransgenic littermates (Center), and fat-1 mice (Right). (B) Colon shortening as a hallmark of DSS-induced colonic damage is reduced in fat-1 mice. ∗, P < 0.01 versus WT DSS-treated animals. (C) Histopathological scores for colonic inflammatory infiltration and epithelial damage in WT and fat-1 mice. ∗, P < 0.01 versus WT DSS. (D) Body weight change from 100% baseline over 8 days in fat-1 mice and WT littermates (n = 6 for each group), 5 days of DSS treatment and 3 days of normal drinking water. ∗, P < 0.05 versus WT DSS; ∗∗, P < 0.01 versus WT DSS. Mice were killed on day 8 (arrow), and samples were taken for further analysis.
Fig. 3.
LC–UV–tandem MS profiles of n-3 PUFA-derived lipid mediators. (A) DHA-derived resolvins and protectins (main pathway products identified were RvD3 and PD1/NPD1). (B) EPA-derived bioactive lipid mediators (identified mediators include RvE1, PGE3, and LTB5). (C) Arachidonic acid-derived bioactive mediators [PGE2, LTB4, and 15-hydroxyeicosatetraenoic acid (15-HETE) as precursor for the n-6 PUFA-derived lipoxin A4 (LXA4)]. (D) Presence of different lipid mediators in colon samples of fat-1 transgenic mice (n = 6) and WT animals (n = 6). ∗∗, P < 0.01; ∗, P < 0.05. Note the different scale for 15-HETE and PGE2 (on the right).
Fig. 4.
Markers of inflammation and mucoprotection. (A) NF-κB activation reflected in p65 ELISA activity shows significant differences in control baselines and in disease between WT and fat-1 mice. ∗, P < 0.05 versus WT DSS; ∗∗, P < 0.05 versus WT control. (B_–_F) Semiquantitative real-time PCR analysis of mRNA expression levels of inflammatory mediators TNFα, inducible NO synthase (iNOS), and IL-1β (B_–_D) and mucoprotective factors Tollip and TFF3 (E and F) in colons from WT and fat-1 mice after DSS exposure and fat-1 control mice, normalized as fold increase to the baseline of WT controls (dashed line). ∗, P < 0.05 versus WT DSS; ∗∗, P < 0.01 versus WT DSS. (G) ZO-1 expression profile. Compared with WT mice without treatment (Left), ZO-1 expression is down-regulated on the luminal epithelial surface in WT mice on day 4 (Center), whereas luminal continuity of expression is sustained in fat-1 mice (Right).
Similar articles
- Omega-3 fatty acids protect from colitis via an Alox15-derived eicosanoid.
Rohwer N, Chiu CY, Huang D, Smyl C, Rothe M, Rund KM, Helge Schebb N, Kühn H, Weylandt KH. Rohwer N, et al. FASEB J. 2021 Apr;35(4):e21491. doi: 10.1096/fj.202002340RR. FASEB J. 2021. PMID: 33710695 - Colitis-associated colon tumorigenesis is suppressed in transgenic mice rich in endogenous n-3 fatty acids.
Nowak J, Weylandt KH, Habbel P, Wang J, Dignass A, Glickman JN, Kang JX. Nowak J, et al. Carcinogenesis. 2007 Sep;28(9):1991-5. doi: 10.1093/carcin/bgm166. Epub 2007 Jul 18. Carcinogenesis. 2007. PMID: 17634405 - Modulation of inflammatory cytokines by omega-3 fatty acids.
Kang JX, Weylandt KH. Kang JX, et al. Subcell Biochem. 2008;49:133-43. doi: 10.1007/978-1-4020-8831-5_5. Subcell Biochem. 2008. PMID: 18751910 Review. - Fish oil feeding attenuates neuroinflammatory gene expression without concomitant changes in brain eicosanoids and docosanoids in a mouse model of Alzheimer's disease.
Hopperton KE, Trépanier MO, James NCE, Chouinard-Watkins R, Bazinet RP. Hopperton KE, et al. Brain Behav Immun. 2018 Mar;69:74-90. doi: 10.1016/j.bbi.2017.11.002. Epub 2017 Nov 3. Brain Behav Immun. 2018. PMID: 29109025 - Omega-3 fatty acids and their lipid mediators: towards an understanding of resolvin and protectin formation.
Weylandt KH, Chiu CY, Gomolka B, Waechter SF, Wiedenmann B. Weylandt KH, et al. Prostaglandins Other Lipid Mediat. 2012 Mar;97(3-4):73-82. doi: 10.1016/j.prostaglandins.2012.01.005. Epub 2012 Feb 3. Prostaglandins Other Lipid Mediat. 2012. PMID: 22326554 Review.
Cited by
- Resolution of Acute Inflammation and the Role of Resolvins in Immunity, Thrombosis, and Vascular Biology.
Sansbury BE, Spite M. Sansbury BE, et al. Circ Res. 2016 Jun 24;119(1):113-30. doi: 10.1161/CIRCRESAHA.116.307308. Circ Res. 2016. PMID: 27340271 Free PMC article. Review. - Endogenously produced n-3 fatty acids protect against ovariectomy induced bone loss in fat-1 transgenic mice.
Banu J, Bhattacharya A, Rahman M, Kang JX, Fernandes G. Banu J, et al. J Bone Miner Metab. 2010 Nov;28(6):617-26. doi: 10.1007/s00774-010-0175-2. J Bone Miner Metab. 2010. PMID: 20393761 - Therapeutic Potential of ω-3 Polyunsaturated Fatty Acids in Human Autoimmune Diseases.
Li X, Bi X, Wang S, Zhang Z, Li F, Zhao AZ. Li X, et al. Front Immunol. 2019 Sep 27;10:2241. doi: 10.3389/fimmu.2019.02241. eCollection 2019. Front Immunol. 2019. PMID: 31611873 Free PMC article. Review. - Identification of specialized pro-resolving mediator clusters from healthy adults after intravenous low-dose endotoxin and omega-3 supplementation: a methodological validation.
Norris PC, Skulas-Ray AC, Riley I, Richter CK, Kris-Etherton PM, Jensen GL, Serhan CN, Maddipati KR. Norris PC, et al. Sci Rep. 2018 Dec 21;8(1):18050. doi: 10.1038/s41598-018-36679-4. Sci Rep. 2018. PMID: 30575798 Free PMC article. Clinical Trial. - Fat-1 Transgene Is Associated With Improved Reproductive Outcomes.
Hohos NM, Cho KJ, Swindle DC, Allshouse AA, Rudolph MC, Skaznik-Wikiel ME. Hohos NM, et al. Endocrinology. 2018 Dec 1;159(12):3981-3992. doi: 10.1210/en.2018-00723. Endocrinology. 2018. PMID: 30403782 Free PMC article.
References
- Podolsky D. K. N. Engl. J. Med. 2002;347:417–429. - PubMed
- Belluzzi A., Brignola C., Campieri M., Pera A., Boschi S., Miglioli M. N. Engl. J. Med. 1996;334:1557–1560. - PubMed
- Endres S., Lorenz R., Loeschke K. Curr. Opin. Clin. Nutr. Metab. Care. 1999;2:117–120. - PubMed
- Stenson W. F., Cort D., Rodgers J., Burakoff R., DeSchryver-Kecskemeti K., Gramlich T. L., Beeken W. Ann. Intern. Med. 1992;116:609–614. - PubMed
- Haeggstrom J. Z. J. Biol. Chem. 2004;279:50639–50642. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 DK040561/DK/NIDDK NIH HHS/United States
- P30 DK043351/DK/NIDDK NIH HHS/United States
- P50-DE016191/DE/NIDCR NIH HHS/United States
- DK43351/DK/NIDDK NIH HHS/United States
- P30 DK040561-11/DK/NIDDK NIH HHS/United States
- P50 DE016191/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases