Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms - PubMed (original) (raw)
. 2006 Jul 25;103(30):11358-63.
doi: 10.1073/pnas.0604517103. Epub 2006 Jul 18.
Svetlana Yanina, Jeffrey S McLean, Kevin M Rosso, Dianne Moyles, Alice Dohnalkova, Terry J Beveridge, In Seop Chang, Byung Hong Kim, Kyung Shik Kim, David E Culley, Samantha B Reed, Margaret F Romine, Daad A Saffarini, Eric A Hill, Liang Shi, Dwayne A Elias, David W Kennedy, Grigoriy Pinchuk, Kazuya Watanabe, Shun'ichi Ishii, Bruce Logan, Kenneth H Nealson, Jim K Fredrickson
Affiliations
- PMID: 16849424
- PMCID: PMC1544091
- DOI: 10.1073/pnas.0604517103
Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms
Yuri A Gorby et al. Proc Natl Acad Sci U S A. 2006.
Abstract
Shewanella oneidensis MR-1 produced electrically conductive pilus-like appendages called bacterial nanowires in direct response to electron-acceptor limitation. Mutants deficient in genes for c-type decaheme cytochromes MtrC and OmcA, and those that lacked a functional Type II secretion pathway displayed nanowires that were poorly conductive. These mutants were also deficient in their ability to reduce hydrous ferric oxide and in their ability to generate current in a microbial fuel cell. Nanowires produced by the oxygenic phototrophic cyanobacterium Synechocystis PCC6803 and the thermophilic, fermentative bacterium Pelotomaculum thermopropionicum reveal that electrically conductive appendages are not exclusive to dissimilatory metal-reducing bacteria and may, in fact, represent a common bacterial strategy for efficient electron transfer and energy distribution.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
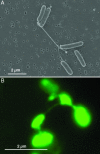
Fig. 1.
Wild-type strain MR-1 taken from an electron-acceptor-limited chemostat operating at low agitation (50 rpm). (A) SEM image of MRI. (B) Epifluorescence micrograph of MR-1 stained with the fluorescent nonspecific protein-binding stain NanoOrange in liquid medium.
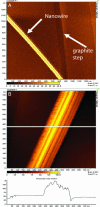
Fig. 2.
STM images of isolated nanowires from wild-type MR-1, with lateral diameter of 100 nm and a topographic height of between 5 and 10 nm. (A) Arrows indicate the location of a nanowire and a step on the graphite substrate. (B) Higher magnification showing ridges and troughs running along the long axis of the structures.
Fig. 3.
Transmission EM images of whole mounts of MR-1 cells incubated in an aqueous suspension of Si-HFO. The Si-HFO was transformed to nanocrystalline magnetite along extracellular features consistent with the dimensions of nanowires.
Fig. 4.
SEM and STM images of nanowires produced by cyanobacteria and methanogenic cocultures. (A) SEM image of Synechocystis sp. PCC 6803 cultivated with CO2 limitation and excess light. (B) STM imagery confirms that the extracellular appendages produced under these conditions are highly electrically conductive, with morphological similarities to nanowires produced by S. oneidensis MR-1. (C) SEM image of P. thermopropionicum and M. thermoautotrophicus (arrow) in methanogenic cocultures showing nanowires connecting the two genera, as reported by Ishii et al. (12). (D) STM images confirm that these nanowires are highly conductive and composed of bundles of individual filaments.
Similar articles
- Regulation of Gene Expression in Shewanella oneidensis MR-1 during Electron Acceptor Limitation and Bacterial Nanowire Formation.
Barchinger SE, Pirbadian S, Sambles C, Baker CS, Leung KM, Burroughs NJ, El-Naggar MY, Golbeck JH. Barchinger SE, et al. Appl Environ Microbiol. 2016 Aug 15;82(17):5428-43. doi: 10.1128/AEM.01615-16. Print 2016 Sep 1. Appl Environ Microbiol. 2016. PMID: 27342561 Free PMC article. - Outer Membrane _c_-Type Cytochromes OmcA and MtrC Play Distinct Roles in Enhancing the Attachment of Shewanella oneidensis MR-1 Cells to Goethite.
Jing X, Wu Y, Shi L, Peacock CL, Ashry NM, Gao C, Huang Q, Cai P. Jing X, et al. Appl Environ Microbiol. 2020 Nov 10;86(23):e01941-20. doi: 10.1128/AEM.01941-20. Print 2020 Nov 10. Appl Environ Microbiol. 2020. PMID: 32978123 Free PMC article. - Electrical transport along bacterial nanowires from Shewanella oneidensis MR-1.
El-Naggar MY, Wanger G, Leung KM, Yuzvinsky TD, Southam G, Yang J, Lau WM, Nealson KH, Gorby YA. El-Naggar MY, et al. Proc Natl Acad Sci U S A. 2010 Oct 19;107(42):18127-31. doi: 10.1073/pnas.1004880107. Epub 2010 Oct 11. Proc Natl Acad Sci U S A. 2010. PMID: 20937892 Free PMC article. - Respiration of metal (hydr)oxides by Shewanella and Geobacter: a key role for multihaem c-type cytochromes.
Shi L, Squier TC, Zachara JM, Fredrickson JK. Shi L, et al. Mol Microbiol. 2007 Jul;65(1):12-20. doi: 10.1111/j.1365-2958.2007.05783.x. Mol Microbiol. 2007. PMID: 17581116 Free PMC article. Review. - Mechanisms of Bacterial Extracellular Electron Exchange.
White GF, Edwards MJ, Gomez-Perez L, Richardson DJ, Butt JN, Clarke TA. White GF, et al. Adv Microb Physiol. 2016;68:87-138. doi: 10.1016/bs.ampbs.2016.02.002. Epub 2016 Mar 24. Adv Microb Physiol. 2016. PMID: 27134022 Review.
Cited by
- Impact of additives on syntrophic propionate and acetate enrichments under high-ammonia conditions.
Pinela E, Schnürer A, Neubeck A, Moestedt J, Westerholm M. Pinela E, et al. Appl Microbiol Biotechnol. 2024 Aug 7;108(1):433. doi: 10.1007/s00253-024-13263-7. Appl Microbiol Biotechnol. 2024. PMID: 39110235 Free PMC article. - Comparative analysis of the influence of BpfA and BpfG on biofilm development and current density in Shewanella oneidensis under oxic, fumarate- and anode-respiring conditions.
Klein EM, Heintz H, Wurst R, Schuldt S, Hähl H, Jacobs K, Gescher J. Klein EM, et al. Sci Rep. 2024 Oct 5;14(1):23174. doi: 10.1038/s41598-024-73474-w. Sci Rep. 2024. PMID: 39369013 Free PMC article. - Flavin electron shuttles dominate extracellular electron transfer by Shewanella oneidensis.
Kotloski NJ, Gralnick JA. Kotloski NJ, et al. mBio. 2013 Jan 15;4(1):e00553-12. doi: 10.1128/mBio.00553-12. mBio. 2013. PMID: 23322638 Free PMC article. - Isolation and Characterization of Electrochemically Active Subsurface Delftia and Azonexus Species.
Jangir Y, French S, Momper LM, Moser DP, Amend JP, El-Naggar MY. Jangir Y, et al. Front Microbiol. 2016 May 23;7:756. doi: 10.3389/fmicb.2016.00756. eCollection 2016. Front Microbiol. 2016. PMID: 27242768 Free PMC article. - Metabolite-enabled mutualistic interaction between Shewanella oneidensis and Escherichia coli in a co-culture using an electrode as electron acceptor.
Wang VB, Sivakumar K, Yang L, Zhang Q, Kjelleberg S, Loo SC, Cao B. Wang VB, et al. Sci Rep. 2015 Jun 10;5:11222. doi: 10.1038/srep11222. Sci Rep. 2015. PMID: 26061569 Free PMC article.
References
- Nealson K. H., Saffarini D. Annu. Rev. Microbiol. 1994;48:311–343. - PubMed
- Nealson K. H., Belz A., McKee B. Antonie Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2002;81:215–222. - PubMed
- Lovley D. R., Coates J. D., Blunt-Harris E. L., Phillips E. J. P., Woodward J. C. Nature. 1996;382:445–448.
- Newman D. K., Kolter R. Nature. 2000;405:94–97. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources