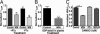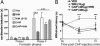The cannabinoid WIN 55,212-2 inhibits transient receptor potential vanilloid 1 (TRPV1) and evokes peripheral antihyperalgesia via calcineurin - PubMed (original) (raw)
The cannabinoid WIN 55,212-2 inhibits transient receptor potential vanilloid 1 (TRPV1) and evokes peripheral antihyperalgesia via calcineurin
Amol M Patwardhan et al. Proc Natl Acad Sci U S A. 2006.
Abstract
Cannabinoids can evoke antihyperalgesia and antinociception at a peripheral site of action. However, the signaling pathways mediating these effects are not clearly understood. We tested the hypothesis that certain cannabinoids directly inhibit peripheral capsaicin-sensitive nociceptive neurons by dephosphorylating and desensitizing transient receptor potential vanilloid 1 (TRPV1) via a calcium calcineurin-dependent mechanism. Application of the cannabinoid WIN 55,212-2 (WIN) to cultured trigeminal (TG) neurons or isolated skin biopsies rapidly and significantly inhibited capsaicin-activated inward currents and neuropeptide exocytosis by a mechanism requiring the presence of extracellular calcium. The inhibitory effect did not involve activation of G protein-coupled cannabinoid receptors, because neither pertussis toxin nor GDPbetaS treatments altered the WIN effect. However, application of WIN-activated calcineurin, as measured by nuclear translocation of the nuclear factor of activated T cells (NFAT)c4 transcription factor, dephosphorylated TRPV1. The WIN-induced desensitization of TRPV1 was mediated by calcineurin, because the application of structurally distinct calcineurin antagonists (calcineurin autoinhibitory peptide and cyclosporine/cyclophilin complex) abolished WIN-induced inhibition of capsaicin-evoked inward currents and neuropeptide exocytosis. This mechanism also contributed to peripheral antinociceptive/antihyperalgesic effects of WIN because pretreatment with the calcineurin antagonist calcineurin autoinhibitory peptide (CAIP) significantly reduced peripherally mediated WIN effects in two behavioral models. Collectively, these data demonstrate that cannabinoids such as WIN directly inhibit TRPV1 functional activities via a calcineurin pathway that represents a mechanism of cannabinoid actions at peripheral sites.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
Fig. 1.
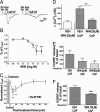
The cannabinoid agonist WIN 55,212-2 (WIN) reversibly inhibits capsaicin responses from nociceptors. (A) Effect of vehicle (VEH) or WIN pretreatment on capsaicin-induced inward current in cultured rat TG neurons. Cultured TG neurons were grown in presence of nerve growth factor (NGF) for 24–48 h. The intact neurons were exposed to VEH/WIN (25 μM) for 3 min, washed for 2 min, and then _I_CAP [0.5 μM capsaicin (CAP) for 40 s] were recorded in whole-cell configuration. Representative traces denote capsaicin current (_I_CAP) after VEH or WIN treatment. (B) Effect of pretreatment with different concentrations of WIN on Icap. Data are normalized to _I_CAP after VEH pretreatment (n = 8–13 cells per condition; ∗∗, P < 0.01, ANOVA with Bonferroni post hoc test). (C) The time course of WIN (25 μM for 3 min, then wash) inhibition of _I_CAP is shown (n = 8–12 cells per condition; ∗, P < 0.05; ∗∗, P < 0.01, ANOVA with Bonferroni post hoc test). (D) Effect of VEH or WIN (25 μM) pretreatment on capsaicin-induced calcium influx in cultured TG neurons. The Fura-loaded cells were exposed to either vehicle or WIN (25 μM) for 3 min. After the WIN-induced calcium influx returned to the basal level, the cells were exposed to capsaicin (0.5 μM for 40 s). The elevation (Δ) in [Ca2+]i in VEH-WIN group denotes the calcium influx evoked by WIN alone over baseline values, and the elevation (Δ) in [Ca2+]i in the VEH-CAP and WIN-CAP groups denotes the CAP-evoked calcium influx over baseline levels after VEH or WIN pretreatment (n = 16–25; ∗∗, P < 0.05, ANOVA with Bonferroni post hoc test). (E) Effect of VEH or WIN pretreatment on capsaicin-induced CGRP release from acutely dissociated rat TG neurons. Freshly isolated and dissociated TG were pretreated with either vehicle or WIN for 7 min, washed, and then pulsed with capsaicin alone (100 μM) for 2 min. Capsaicin-evoked iCGRP release was measured by radioimmunoassay (RIA) and is represented as percent of basal release (n = 8; ∗, P < 0.05, ANOVA with Bonferroni post hoc test). (F) Effect of VEH or WIN pretreatment on capsaicin-induced CGRP release from acutely isolated hindpaw skin. Freshly isolated hindpaw skin was pretreated with VEH or WIN (10 μM), washed (10 min), and then pulsed with capsaicin alone (100 μM) for 2 min. Capsaicin-evoked iCGRP release was measured by RIA and is represented as percent of basal release (n = 6; ∗∗, P < 0.01, Student’s t test).
Fig. 2.
WIN inhibition of capsaicin-evoked responses is independent of G protein activation. (A) Effect of pretreatment of TG neurons with pertussis toxin (PTx). Cultured TG neurons were grown in the presence or absence of PTx (500 ng/ml, 24–36 h). Vehicle (VEH) or WIN was applied with _I_CAP as described in the legend for Fig. 1. (n = 7–10 cells; ∗∗, P < 0.01, ANOVA with Bonferroni post hoc test). (B) Effect of trapping of G protein activities by dialyzing neurons (5–8 min) with GDP-βS (2.5 mM) on WIN inhibition of _I_CAP (n = 8–9 cells; ∗∗, P < 0.01, Student’s t test). (C) Positive control experiments, demonstrating the inhibitory effect of pertussis toxin or GDP-βS on [
d
-Ala2,_N_-MePhe4,Gly5-ol]enkephalin (DAMGO) (1 μM) inhibition of high voltage-activated calcium currents (HVA _I_Ca, n = 8–11 cells; ∗∗, P < 0.01, ANOVA with Bonferroni post hoc test).
Fig. 3.
WIN inhibition of capsaicin-evoked responses is dependent on activation of the calcium-calcineurin pathway. (A) Evaluation of calcium dependency of WIN and CAP for inhibition of _I_CAP. Electrophysiology experiments were conducted as described in the legend to Fig. 1, except that WIN and CAP treatment was administered in the presence of either 2 mM calcium (normal) or 0 mM calcium in the external solution. Representative traces showing capsaicin/WIN desensitization of _I_CAP in the presence or absence of external calcium. (B) Graphical representation of traces in A demonstrating the calcium dependency of WIN and CAP for inhibition of _I_CAP (n = 14 cells; ∗∗, P < 0.01; ∗∗∗, P < 0.001, ANOVA with Bonferroni post hoc test). (C) Effect of WIN pretreatment in calcium-free buffer on WIN inhibition of capsaicin evoked iCGRP release from isolated hindpaw skin (n = 12; ∗∗, P < 0.01, ANOVA with Bonferroni post hoc test). The superfusion experiments were conducted as described in the legend to Fig. 1, except that WIN pretreatment was administered in the presence of either 2 mM calcium (normal) or 0 mM calcium in the external solution; all groups then were returned to 2 mM calcium concentrations for the 10 min wash and during the application of capsaicin. (D) Evaluation of the calcineurin dependency of WIN and CAP for inhibition of _I_CAP. Electrophysiology experiments were conducted as described in the legend to Fig. 1, except that WIN or CAP treatment was administered in presence of CAIP (100 μg) or the complex of 51 nM cyclosporine/100 nM cyclophilin. Representative traces are shown. (E) Graphical representation of traces in D demonstrating the effect of inhibition of calcineurin on WIN and CAP inhibition of _I_CAP (n = 6–15 cells; ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001, ANOVA with Bonferroni post hoc test). (F) Effect of WIN pretreatment in the presence of CAIP (100 μM) on WIN inhibition of capsaicin-evoked iCGRP release from isolated hindpaw skin (n = 6; ∗, P < 0.01, ANOVA with Bonferroni post hoc test).
Fig. 4.
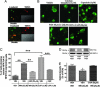
WIN activates calcineurin in TG neurons and dephosphorylates TRPV1. (A) Calcineurin subunits are coexpressed with TRPV1. The colocalization of calcineurin A subunit (Upper) and B subunit (Lower) with TRPV1 is demonstrated in the respective panels. (B) The effect of various treatments on the nuclear translocation of NFATc4 (calcineurin activation). Cultured TG neurons were exposed to vehicle, ionomycin (1 μM), capsaicin (1 μM), WIN (25 μM), or WIN+CAIP (25 μM/50 μM), and immunohistochemistry was performed by using an antibody against NFATc4. (C) Graphical representation of the percent of NFATc4 positive neurons showing nuclear translocation of NFAFc4 after treatment with VEH/VEH, VEH/WIN (25 μM), CAIP (50 μM)/WIN, VEH/CAP (1 μM), or CAIP (50 μM)/CAP (n = 4 independent cultures assessed by blinded observer; n = 152–180 cells per condition; ∗∗, P < 0.01, ANOVA with Bonferroni post hoc test). (D) Effect of WIN and CAIP/WIN treatment on TRPV1 phosphorylation. Cultured TG neurons (6 ganglia per 10-cm plate) were treated with VEH, WIN (25 μM), or CAIP (50 μM)+WIN. A representative Western blot demonstrating phosphothreonine content (Upper) in TRPV1 immunoprecipitated TG lysates (Lower). (E) Quantification of multiple experiments performed as described in D. The band density was normalized to total TRPV1 protein (n = 3 independent cultures; ∗, P < 0.05, ANOVA with Bonferroni post hoc test).
Fig. 5.
WIN inhibition of orofacial nocifensive behavior and hindpaw hyperalgesia is dependent on activation of calcineurin. (A) After habituation to the testing chamber, lightly restrained rats were injected in the right vibrissal pad with VEH, WIN (10 μg), CAIP (400 μg), or CAIP/WIN along with 5% formalin. In a separate group of animals, the same dose (10 μg) of WIN was injected systemically (SYS, s.c.) before formalin injection. The results are represented as the mean number of seconds the animal displayed nocifensive behavior as measured by blinded observers (n = 6 animals per group; ∗∗∗, P < 0.001, two-way ANOVA with Bonferroni post hoc test). (B) Lightly restrained rats were injected with VEH, WIN (10 μg), CAIP (400 μg), or WIN+CAIP in the right hindpaw. The same paws were injected with capsaicin (10 μg) 15 min later. The thermal withdrawal latencies were measured 5 and 10 min after capsaicin injection by blinded observers. Data are depicted as paw withdrawal latency in seconds. To evaluate the development of hyperalgesia, post-capsaicin withdrawal latencies were compared with the basal withdrawal latencies (n = 6–10 animals per group, two-way ANOVA with Bonferroni post hoc test; ###, P < 0.001; ##, P < 0.01). To evaluate the WIN-evoked antihyperalgesia and its modulation by CAIP, the statistical comparison was made with respect to the VEH-CAP group (n = 6–10 animals per group; ∗∗∗, P < 0.001, two-way ANOVA with Bonferroni post hoc test).
Similar articles
- Role of ionotropic cannabinoid receptors in peripheral antinociception and antihyperalgesia.
Akopian AN, Ruparel NB, Jeske NA, Patwardhan A, Hargreaves KM. Akopian AN, et al. Trends Pharmacol Sci. 2009 Feb;30(2):79-84. doi: 10.1016/j.tips.2008.10.008. Epub 2008 Dec 11. Trends Pharmacol Sci. 2009. PMID: 19070372 Free PMC article. Review. - Cannabinoid WIN 55,212-2 inhibits TRPV1 in trigeminal ganglion neurons via PKA and PKC pathways.
Wang W, Cao X, Liu C, Liu L. Wang W, et al. Neurol Sci. 2012 Feb;33(1):79-85. doi: 10.1007/s10072-011-0620-6. Epub 2011 May 17. Neurol Sci. 2012. PMID: 21584737 - Cannabinoid WIN 55,212-2 regulates TRPV1 phosphorylation in sensory neurons.
Jeske NA, Patwardhan AM, Gamper N, Price TJ, Akopian AN, Hargreaves KM. Jeske NA, et al. J Biol Chem. 2006 Oct 27;281(43):32879-90. doi: 10.1074/jbc.M603220200. Epub 2006 Sep 5. J Biol Chem. 2006. PMID: 16954222 Free PMC article. - Cannabinoid receptor-independent actions of the aminoalkylindole WIN 55,212-2 on trigeminal sensory neurons.
Price TJ, Patwardhan A, Akopian AN, Hargreaves KM, Flores CM. Price TJ, et al. Br J Pharmacol. 2004 May;142(2):257-66. doi: 10.1038/sj.bjp.0705778. Br J Pharmacol. 2004. PMID: 15155534 Free PMC article. - TRPV1: A Common Denominator Mediating Antinociceptive and Antiemetic Effects of Cannabinoids.
Louis-Gray K, Tupal S, Premkumar LS. Louis-Gray K, et al. Int J Mol Sci. 2022 Sep 2;23(17):10016. doi: 10.3390/ijms231710016. Int J Mol Sci. 2022. PMID: 36077412 Free PMC article. Review.
Cited by
- Role of ionotropic cannabinoid receptors in peripheral antinociception and antihyperalgesia.
Akopian AN, Ruparel NB, Jeske NA, Patwardhan A, Hargreaves KM. Akopian AN, et al. Trends Pharmacol Sci. 2009 Feb;30(2):79-84. doi: 10.1016/j.tips.2008.10.008. Epub 2008 Dec 11. Trends Pharmacol Sci. 2009. PMID: 19070372 Free PMC article. Review. - Combined analgesics in (headache) pain therapy: shotgun approach or precise multi-target therapeutics?
Straube A, Aicher B, Fiebich BL, Haag G. Straube A, et al. BMC Neurol. 2011 Mar 31;11:43. doi: 10.1186/1471-2377-11-43. BMC Neurol. 2011. PMID: 21453539 Free PMC article. Review. - Promising cannabinoid-based therapies for Parkinson's disease: motor symptoms to neuroprotection.
More SV, Choi DK. More SV, et al. Mol Neurodegener. 2015 Apr 8;10:17. doi: 10.1186/s13024-015-0012-0. Mol Neurodegener. 2015. PMID: 25888232 Free PMC article. Review. - Transient receptor potential TRPA1 channel desensitization in sensory neurons is agonist dependent and regulated by TRPV1-directed internalization.
Akopian AN, Ruparel NB, Jeske NA, Hargreaves KM. Akopian AN, et al. J Physiol. 2007 Aug 15;583(Pt 1):175-93. doi: 10.1113/jphysiol.2007.133231. Epub 2007 Jun 21. J Physiol. 2007. PMID: 17584831 Free PMC article. - Hemopressin is an inverse agonist of CB1 cannabinoid receptors.
Heimann AS, Gomes I, Dale CS, Pagano RL, Gupta A, de Souza LL, Luchessi AD, Castro LM, Giorgi R, Rioli V, Ferro ES, Devi LA. Heimann AS, et al. Proc Natl Acad Sci U S A. 2007 Dec 18;104(51):20588-93. doi: 10.1073/pnas.0706980105. Epub 2007 Dec 12. Proc Natl Acad Sci U S A. 2007. PMID: 18077343 Free PMC article.
References
- Matsuda L. A., Lolait S. J., Brownstein M. J., Young A. C., Bonner T. I. Nature. 1990;346:561–564. - PubMed
- Munro S., Thomas K. L., Abu-Shaar M. Nature. 1993;365:61–65. - PubMed
- Zygmunt P. M., Petersson J., Andersson D. A., Chuang H., Sorgard M., Di Marzo V., Julius D., Hogestatt E. D. Nature. 1999;400:452–457. - PubMed
- Watanabe H., Vriens J., Prenen J., Droogmans G., Voets T., Nilius B. Nature. 2003;424:434–438. - PubMed
- Jordt S. E., Bautista D. M., Chuang H. H., McKemy D. D., Zygmunt P. M., Hogestatt E. D., Meng I. D., Julius D. Nature. 2004;427:260–265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-DA19585/DA/NIDA NIH HHS/United States
- F32 DE016500/DE/NIDCR NIH HHS/United States
- F32-DE016500/DE/NIDCR NIH HHS/United States
- R01 DA019585/DA/NIDA NIH HHS/United States
- F32 DE016500-01/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous