The role of myosin II motor activity in distributing myosin asymmetrically and coupling protrusive activity to cell translocation - PubMed (original) (raw)
The role of myosin II motor activity in distributing myosin asymmetrically and coupling protrusive activity to cell translocation
John Kolega. Mol Biol Cell. 2006 Oct.
Abstract
Nonmuscle myosin IIA and IIB distribute preferentially toward opposite ends of migrating endothelial cells. To understand the mechanism and function of this behavior, myosin II was examined in cells treated with the motor inhibitor, blebbistatin. Blebbistatin at > or = 30 microM inhibited anterior redistribution of myosin IIA, with 100 microM blebbistatin causing posterior accumulation. Posterior accumulation of myosin IIB was unaffected. Time-lapse cinemicrography showed myosin IIA entering lamellipodia shortly after their formation, but failing to move into lamellipodia in blebbistatin. Thus, myosin II requires motor activity to move forward onto F-actin in protrusions. However, this movement is inhibited by myosin filament assembly, because whole myosin was delayed relative to a tailless fragment. Inhibiting myosin's forward movement reduced coupling between protrusive activity and translocation of the cell body: In untreated cells, body movement followed advancing lamellipodia, whereas blebbistatin-treated cells extended protrusions without displacement of the body or with a longer delay before movement. Anterior cytoplasm of blebbistatin-treated cells contained disorganized bundles of parallel microfilaments, but anterior F-actin bundles in untreated cells were mostly oriented perpendicular to movement. Myosin II may ordinarily move anteriorly on actin filaments and pull crossed filaments into antiparallel bundles, with the resulting realignment pulling the cell body forward.
Figures
Figure 1.
Asymmetric distribution of F-actin and myosin II in migrating endothelial cells. Monolayer cultures of BAECs were wounded in the absence (A–C) or presence (D–F) of 50 μM blebbistatin and were fixed 1 h later. Cells were triple-stained for F-actin (A and D), myosin IIA (B and E), and myosin IIB (C and F). In all panels, the wound is at the top. Brackets in A–C mark the extended anterior cytoplasm where there is an abundance of myosin IIA and little myosin IIB; compare bracketed protrusions in D–F. Note the bright accumulations of myosin IIA in the rear of blebbistatin-treated cells (E, arrows).
Figure 2.
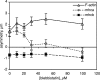
Dose dependence of blebbistatin effects on cytoskeletal asymmetry. The asymmetric distributions of F-actin, myosin IIA, and myosin IIB 1 h after wounding an endothelial monolayer were measured by determining their displacements relative to the center of mass of protein in the cell as described in Materials and Methods. The magnitude of the asymmetry indicates the displacement of the center of mass toward the wound, with a negative asymmetry indicating accumulation away from the wound (i.e., toward the rear of the cell). Each point represents the mean of measurements from 20 to 40 randomly selected cells; error bars, 1 SD.
Figure 3.
Inhibition of anterior distribution of myosin IIB by blebbistatin. Monolayer cultures of BAECs were wounded in 5 μM Y27632 alone (A and B) or 5 μM Y27632 plus 50 μM blebbistatin (C and D) and fixed 1 h later. Cells were stained for protein with lysine-reactive cy5 (A and C) and for myosin IIB by immunofluorescence (B and D). In Y27632, myosin IIB distributed throughout the cytoplasm, extending well into the anterior cytoplasm and very close to the leading edge (B, arrows). In blebbistatin-treated cells, myosin IIB be was largely excluded from the front of the cell (C and D, brackets) and accumulated in the tail (D, arrows). The asymmetries of F-actin and myosin IIB asymmetry were measured 1 h after wounding in 5 μM Y-27632 and various concentrations of blebbistatin (E). Each point represents the mean of measurements from 20 to 40 randomly selected cells; error bars, 1 SD. Note the positive asymmetry of myosin IIB in Y27632 without blebbistatin (compare with Figures 1 and 2) and the loss of myosin IIB asymmetry at ≥20 μM blebbistatin.
Figure 4.
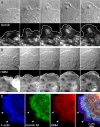
Myosin IIA dynamics in migrating cells during blebbistatin treatment. Confluent monolayers of BAECs were scrape-wounded, and cells along the wound edge were microinjected with TMR-myosin IIA 60 min after wounding. After allowing TMR-myosin IIA to distribute in the cells for 3–6 h, pairs of transmitted-light and fluorescence images were acquired at 2-min intervals. Time after the beginning of imaging is indicated in min:sec in each frame. These micrographs show the fluorescence images at selected intervals, with the edge of the cell as determined from transmitted-light images indicated by the white outlines. At t = 36:00, the cells were perfused with 50 μM blebbistatin. Double-headed arrows indicate regions of cytoplasm that have advanced with no detectable entry of TMR-myosin IIA.
Figure 5.
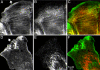
Comparison of myosin IIA and HMM at the front of migrating cells. BAECs at a wound edge were microinjected with rhodamine-labeled full-length myosin II (A) or rhodamine-labeled HMM (B and C). (A and B) Dynamic behavior of myosin II and HMM. Two hours after microinjection, fluorescent cells were imaged at 30-s intervals using DIC optics and fluorescence (the top and bottom rows, respectively, in each sequence). Selected images, 2 min apart, are shown. Note that in A full-length myosin II lags behind the advancing edge, indicated by the white line in the fluorescence images, whereas HMM follows the edge very closely (B). (C) Colocalization of myosin IIA and HMM. Two hours after microinjection, cells were fixed and stained for F-actin (blue) and myosin IIA (green); rhodamine-HMM fluorescence is shown in red. Arrowheads indicate the position of the leading edge. All three fluorescent images are overlaid in the right panel, showing HMM and F-actin alone (purple) at the front of the cell and myosin II, HMM, and F-actin present together (white) in the more proximal lamella.
Figure 6.
F-actin organization in blebbistatin-treated cells. BAECs migrating at a wound edge for 1 h in the absence (A–C) or presence (D–F) of 100 μM blebbistatin were double-stained with for F-actin (A and D) and myosin IIA (B and E). Color overlays of F-actin in green and myosin IIA in red are shown in C and F. White arrows in A and D indicate the direction of cell spreading, with the nucleus of the cell located on the right-hand side of the lower edge of the image in both cases. Note the numerous, long, F-actin bundles lying perpendicular to the direction of movement in untreated cells. Myosin IIA is organized in periodic stripes across these bundles. In 100 μM blebbistatin, F-actin-rich “ruffles” (D, black arrows) project from the dorsal surface just behind the leading edge. F-actin bundles are short and isotropic and lack myosin IIA striation.
Figure 7.
Actin filament orientation in blebbistatin-induced bundles. Cells were treated with 100 μM blebbistatin for 60 min, then permeabilized, decorated with HMM, fixed, negative stained, and viewed as whole mounts. This electron micrograph shows a slightly splayed region of a microfilament bundle located ∼3 μm proximal to the cell's advancing edge, which was to the left of the photographed region. White arrows indicate the orientation of HMM arrowheads on the individual filaments. All of the filaments are oriented with their plus (+, barbed) ends toward the leading edge of the cell.
Figure 8.
Inhibition of oriented migration by blebbistatin. Wounds were created in confluent monolayers of BAECs in the presence (bottom panels) or absence (top panels) of 100 μM blebbistatin, and migration of cells at the wound edge was followed by time-lapse micrography. All cells at the wound edge rapidly formed broad lamellipodia, which extended toward the newly created empty space for the first hour after wounding in both blebbistatin-treated and untreated cells (arrowheads, t = 1 h). In the absence of blebbistatin, cells migrated into the wound, traversing several cell diameters over the next 2 h, with lamellipodia persistently oriented toward the wound. In blebbistatin, the wound edge advanced very little after 1 h, and cells extended many lamellipodia laterally and away from the wound (arrowheads, t = 2 h).
Figure 9.
Effects of blebbistatin on nuclear and leading-edge movements. Wounds were created in confluent monolayers of BAECs in normal culture medium (A) or in the presence of 100 μM blebbistatin (B). Time-lapse images were recorded, and the positions of nuclei and leading edges of cells along the wound were tracked for 500 min. Distance was measured as the displacement toward the wound of the most anterior edge of the cell (−) and of the center of the nucleus (○) relative to its position immediately before wounding. Each point is the average of 10 measurements from 10 different cells along the same wound, and the results shown here are representative of six separate experiments. Steeper slopes in control wounds indicate more rapid movement of the leading edge and the nucleus. Note that after spreading rapidly for the first 2–3 h after wounding, the leading edge advanced very poorly in blebbistatin-treated wounds. Double-headed arrows indicate the average distance between the nucleus and leading edge when nuclear movement began, which is longer and occurs later in blebbistatin than in controls.
Figure 10.
Protrusive activity and nuclear displacement during random migration. Subconfluent BAECs were imaged at 5-min intervals for 2 h in the presence (C and D) or absence (A and B) of 100 μM blebbistatin. Top panels (A and C) show the area covered by new protrusions (▴, solid line) and the distance traveled by the nucleus (○, dashed line) during each 5-min interval for a typical single cell during 2 h of observation. In control cells, large protrusions were frequently followed by a large displacement of the nucleus within the next 5–10 min (arrows in A). In contrast, large nuclear movements in the presence of blebbistatin often preceded the formation of large protrusions (arrows in B). Bottom panels (B and D) show the distance moved by the nucleus as a function of the amount of protrusion that occurred during the preceding 5-min interval; dashed lines indicate the best linear fit to the data.
Figure 11.
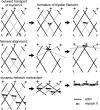
A model for myosin II behavior in anterior cytoplasm of a migrating cell. Single myosin II molecules (small arrows) transport toward the cell's leading edge by moving toward the plus end of recently assembled actin filaments (gray filaments in top left illustration). Where actin filaments cross, myosins can form bipolar filaments that attempt to move outward on two different filaments (top right). Continued plus-directed movement of the bipolar myosin pulls the two crossed actin filaments into antiparallel alignment (middle row). Many myosins acting on multiple actin-filament pairs causes contraction of the network perpendicular to the + → − axis of actin assembly (bottom row).
Similar articles
- Functions of nonmuscle myosin II in assembly of the cellular contractile system.
Shutova M, Yang C, Vasiliev JM, Svitkina T. Shutova M, et al. PLoS One. 2012;7(7):e40814. doi: 10.1371/journal.pone.0040814. Epub 2012 Jul 13. PLoS One. 2012. PMID: 22808267 Free PMC article. - Specificity of blebbistatin, an inhibitor of myosin II.
Limouze J, Straight AF, Mitchison T, Sellers JR. Limouze J, et al. J Muscle Res Cell Motil. 2004;25(4-5):337-41. doi: 10.1007/s10974-004-6060-7. J Muscle Res Cell Motil. 2004. PMID: 15548862 - Nonmuscle myosin II is responsible for maintaining endothelial cell basal tone and stress fiber integrity.
Goeckeler ZM, Bridgman PC, Wysolmerski RB. Goeckeler ZM, et al. Am J Physiol Cell Physiol. 2008 Oct;295(4):C994-1006. doi: 10.1152/ajpcell.00318.2008. Epub 2008 Aug 13. Am J Physiol Cell Physiol. 2008. PMID: 18701651 Free PMC article. - The heavy chain has its day: regulation of myosin-II assembly.
Dulyaninova NG, Bresnick AR. Dulyaninova NG, et al. Bioarchitecture. 2013 Jul-Aug;3(4):77-85. doi: 10.4161/bioa.26133. Bioarchitecture. 2013. PMID: 24002531 Free PMC article. Review. - Mammalian nonmuscle myosin II comes in three flavors.
Shutova MS, Svitkina TM. Shutova MS, et al. Biochem Biophys Res Commun. 2018 Nov 25;506(2):394-402. doi: 10.1016/j.bbrc.2018.03.103. Epub 2018 Mar 17. Biochem Biophys Res Commun. 2018. PMID: 29550471 Free PMC article. Review.
Cited by
- Myosin IIA-related Actomyosin Contractility Mediates Oxidative Stress-induced Neuronal Apoptosis.
Wang Y, Xu Y, Liu Q, Zhang Y, Gao Z, Yin M, Jiang N, Cao G, Yu B, Cao Z, Kou J. Wang Y, et al. Front Mol Neurosci. 2017 Mar 14;10:75. doi: 10.3389/fnmol.2017.00075. eCollection 2017. Front Mol Neurosci. 2017. PMID: 28352215 Free PMC article. - Protrusion and actin assembly are coupled to the organization of lamellar contractile structures.
Lim JI, Sabouri-Ghomi M, Machacek M, Waterman CM, Danuser G. Lim JI, et al. Exp Cell Res. 2010 Aug 1;316(13):2027-41. doi: 10.1016/j.yexcr.2010.04.011. Epub 2010 Apr 18. Exp Cell Res. 2010. PMID: 20406634 Free PMC article. - Different modes of growth cone collapse in NG 108-15 cells.
Rauch P, Heine P, Goettgens B, Käs JA. Rauch P, et al. Eur Biophys J. 2013 Aug;42(8):591-605. doi: 10.1007/s00249-013-0907-z. Epub 2013 May 4. Eur Biophys J. 2013. PMID: 23644679 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources