The glutamate-aspartate transporter GLAST mediates glutamate uptake at inner hair cell afferent synapses in the mammalian cochlea - PubMed (original) (raw)
The glutamate-aspartate transporter GLAST mediates glutamate uptake at inner hair cell afferent synapses in the mammalian cochlea
Elisabeth Glowatzki et al. J Neurosci. 2006.
Abstract
Ribbon synapses formed between inner hair cells (IHCs) and afferent dendrites in the mammalian cochlea can sustain high rates of release, placing strong demands on glutamate clearance mechanisms. To investigate the role of transporters in glutamate removal at these synapses, we made whole-cell recordings from IHCs, afferent dendrites, and glial cells adjacent to IHCs [inner phalangeal cells (IPCs)] in whole-mount preparations of rat organ of Corti. Focal application of the transporter substrate D-aspartate elicited inward currents in IPCs, which were larger in the presence of anions that permeate the transporter-associated anion channel and blocked by the transporter antagonist D,L-threo-beta-benzyloxyaspartate. These currents were produced by glutamate-aspartate transporters (GLAST) (excitatory amino acid transporter 1) because they were weakly inhibited by dihydrokainate, an antagonist of glutamate transporter-1 (excitatory amino acid transporter 2) and were absent from IPCs in GLAST-/- cochleas. Furthermore, D-aspartate-induced currents in outside-out patches from IPCs exhibited larger steady-state currents than responses elicited by L-glutamate, a prominent feature of GLAST, and examination of cochlea from GLAST-Discosoma red (DsRed) promoter reporter mice revealed that DsRed expression was restricted to IPCs and other supporting cells surrounding IHCs. Saturation of transporters by photolysis of caged D-aspartate failed to elicit transporter currents in IHCs, as did local application of D-aspartate to afferent terminals, indicating that neither presynaptic nor postsynaptic membranes are major sites for glutamate removal. These data indicate that GLAST in supporting cells is responsible for transmitter uptake at IHC afferent synapses.
Figures
Figure 1.
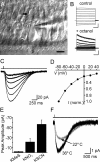
Glutamate transporter currents in cochlear IPCs. A, Infrared-DIC image from a 5-d-old apical cochlear turn. Black arrow, IPC soma; white arrow, triangular-shaped end of IPC phalynx contacting the cuticular plate. Scale bar, 10 μm. B, Responses of IPC to voltage steps (−60 to 140 mV), in control and in 1 m
m
octanol. _V_m = −90 mV. Calibration: top, 4 nA, 50 ms; bottom, 100 pA, 50 ms. C, Inward currents elicited in an IPC in response to 300 μ
m d
-aspartate. The top trace shows the duration of application. D, Current-to-voltage relationship of
d
-aspartate-evoked currents in IPCs (n = 10). Each current was normalized to the peak amplitude recorded at −80 mV. B–D were recorded with a KNO3-based pipette solution. E, Average amplitude of
d
-aspartate-evoked currents recorded at _V_m of −80 mV with different intracellular anions. F,
d
-Aspartate-evoked transporter currents recorded at room temperature (22°C) and at near-physiological temperature (36°C) (_V_m = −80 mV, KMES-based pipette solution).
Figure 2.
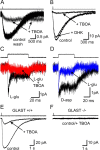
GLAST is the primary glutamate transporter in inner phalangeal cells. A, Glutamate transporter currents recorded in an IPC in response to 500 μ
m d
-aspartate, in the presence and absence of 200 μ
m
TBOA (KMES-based pipette solution). _V_m = −80 mV. B,
d
-Aspartate-evoked transporter currents recorded from an IPC in control, DHK (300 μ
m
), or TBOA (300 μ
m
) (_V_m = −80 mV, KNO3-based pipette solution). C,
l
-Glutamate (10 m
m
)-evoked transporter currents recorded from an outside-out patch from an IPC in control and with 300 μ
m
TBOA. _V_m = −110 mV. D, Glutamate transporter currents recorded from an IPC outside-out patch in response to either 2 m
m l
-glutamate or 2 m
m d
-aspartate. _V_m = −110 mV. In C and D, the traces at the top show the duration of glutamate application. E, F, Glutamate transporter currents recorded from GLAST+/+ and GLAST−/− mice IPCs in response to
d
-aspartate (500 μ
m
), both with and without 300 μ
m
TBOA. _V_m = −80 mV. A–F, KNO3-based pipette solution.
Figure 3.
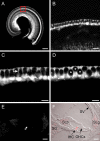
Visualization of GLAST promoter activity in GLAST–DsRed mice. A, DsRed fluorescence in a whole-mount apical turn; a narrow band of labeling was observed in the IHC area (red box). Scale bar, 500 μm. B, Region of the area outlined in A shown at higher magnification. Scale bar, 50 μm. C, D, Images taken at different focal planes of the IHC area. Arrow in C points to a labeled phalynx of an IPC. Asterisks in D mark unlabeled IHCs surrounded by DsRed+ supporting cells. Scale bars, 25 μm. E, DsRed fluorescence in a 14-μm-thick cochlear section. F, Merged fluorescence and DIC image of the section shown in E. In addition to intense labeling around the IHC, satellite cells in the spiral ganglion (SG) and cells in the spiral limbus (SLb) and spiral ligament (SLg) are labeled. SV, Stria vascularis. Scale bars, 50 μm. All images from apical turns of postnatal day 9 GLAST–DsRed BAC mouse cochleas.
Figure 4.
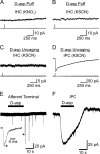
IHCs and their afferent fibers do not exhibit glutamate transporter currents. A, B, Responses of IHCs to 500 μ
m d
-aspartate. A, KNO3-based pipette solution (_V_m = −88 mV). B, KSCN-based pipette solution (_V_m = −70 mV). C, D, Comparison of IHC and IPC responses to UV photolysis of caged
d
-aspartate (MNI-
d
-aspartate, 500 μ
m
). IHC, _V_m = −70 mV; IPC, _V_m = −80 mV. C, D, KSCN-based internal solution. E, F, Comparison of afferent fiber and IPC responses to 500 μ
m d
-aspartate applied locally using a flow pipette. EPSCs appeared spontaneously in afferent fiber recordings; a single event (*) is shown on an expanded timescale. _V_m = −90 mV. KSCN-based pipette solution.
Similar articles
- Distribution of the glutamate/aspartate transporter GLAST in relation to the afferent synapses of outer hair cells in the guinea pig cochlea.
Furness DN, Hulme JA, Lawton DM, Hackney CM. Furness DN, et al. J Assoc Res Otolaryngol. 2002 Sep;3(3):234-47. doi: 10.1007/s101620010064. Epub 2001 Dec 21. J Assoc Res Otolaryngol. 2002. PMID: 12382100 Free PMC article. - Identification of a glutamate/aspartate transporter in the rat cochlea.
Li HS, Niedzielski AS, Beisel KW, Hiel H, Wenthold RJ, Morley BJ. Li HS, et al. Hear Res. 1994 Aug;78(2):235-42. doi: 10.1016/0378-5955(94)90029-9. Hear Res. 1994. PMID: 7527019 - Glutamate transporters in the guinea-pig cochlea: partial mRNA sequences, cellular expression and functional implications.
Rebillard G, Ruel J, Nouvian R, Saleh H, Pujol R, Dehnes Y, Raymond J, Puel JL, Devau G. Rebillard G, et al. Eur J Neurosci. 2003 Jan;17(1):83-92. doi: 10.1046/j.1460-9568.2003.02429.x. Eur J Neurosci. 2003. PMID: 12534971 - [Role of glutamate transporters in excitatory synapses in cerebellar Purkinje cells].
Ozawa S. Ozawa S. Brain Nerve. 2007 Jul;59(7):669-76. Brain Nerve. 2007. PMID: 17663137 Review. Japanese. - Molecular organization of a type of peripheral glutamate synapse: the afferent synapses of hair cells in the inner ear.
Ottersen OP, Takumi Y, Matsubara A, Landsend AS, Laake JH, Usami S. Ottersen OP, et al. Prog Neurobiol. 1998 Feb;54(2):127-48. doi: 10.1016/s0301-0082(97)00054-3. Prog Neurobiol. 1998. PMID: 9481795 Review.
Cited by
- Codon Usage Bias: A Potential Factor Affecting VGLUT Developmental Expression and Protein Evolution.
Zhao Y, Zhang Y, Feng J, He Z, Li T. Zhao Y, et al. Mol Neurobiol. 2024 Sep 21. doi: 10.1007/s12035-024-04426-8. Online ahead of print. Mol Neurobiol. 2024. PMID: 39305444 - Expression of Atoh1, Gfi1, and Pou4f3 in the mature cochlea reprograms nonsensory cells into hair cells.
McGovern MM, Hosamani IV, Niu Y, Nguyen KY, Zong C, Groves AK. McGovern MM, et al. Proc Natl Acad Sci U S A. 2024 Jan 30;121(5):e2304680121. doi: 10.1073/pnas.2304680121. Epub 2024 Jan 24. Proc Natl Acad Sci U S A. 2024. PMID: 38266052 Free PMC article. - Age-related changes in P2Y receptor signalling in mouse cochlear supporting cells.
Hool SA, Jeng JY, Jagger DJ, Marcotti W, Ceriani F. Hool SA, et al. J Physiol. 2023 Oct;601(19):4375-4395. doi: 10.1113/JP284980. Epub 2023 Sep 16. J Physiol. 2023. PMID: 37715703 Free PMC article. - Cochlear transcript diversity and its role in auditory functions implied by an otoferlin short isoform.
Liu H, Liu H, Wang L, Song L, Jiang G, Lu Q, Yang T, Peng H, Cai R, Zhao X, Zhao T, Wu H. Liu H, et al. Nat Commun. 2023 May 29;14(1):3085. doi: 10.1038/s41467-023-38621-3. Nat Commun. 2023. PMID: 37248244 Free PMC article.
References
- Basile AS, Huang JM, Xie C, Webster D, Berlin C, Skolnick P (1996). N-methyl-d-aspartate antagonists limit aminoglycoside antibiotic-induced hearing loss. Nat Med 2:1338–1343. - PubMed
- Bergles DE, Jahr CE (1997). Synaptic activation of glutamate transporters in hippocampal astrocytes. Neuron 19:1297–1308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DC06476/DC/NIDCD NIH HHS/United States
- R01 GM053395/GM/NIGMS NIH HHS/United States
- P30D005211/PHS HHS/United States
- R01 GM053395-11A1/GM/NIGMS NIH HHS/United States
- NS44261/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases