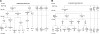Stroke Prevention Trial in Sickle Cell Anemia (STOP): extended follow-up and final results - PubMed (original) (raw)
Randomized Controlled Trial
Stroke Prevention Trial in Sickle Cell Anemia (STOP): extended follow-up and final results
Margaret T Lee et al. Blood. 2006.
Abstract
The Stroke Prevention Trial in Sickle Cell Anemia (STOP) was a randomized trial to evaluate whether chronic transfusion could prevent initial stroke in children with sickle-cell anemia at high risk as determined by transcranial Doppler (TCD). The trial demonstrated a large benefit of transfusion and was halted early. After termination of the trial, patients participated in a post-trial follow-up study. More patients in the transfusion group (70%) elected transfusion for primary stroke prevention compared with those on standard care (45%). Six patients with persistently abnormal TCD results developed stroke. A minority with initially abnormal TCD results remained stroke-free without transfusion. Except for lower baseline and follow-up TCD velocities compared with those with stroke, no predictive features of this apparent lower-risk subgroup could be determined. TCD results at last testing in 108 patients that did not have stroke were: normal (44.4%), conditional (26.9%), abnormal (22.2%), and inadequate (6.5%). Patients on transfusion were more likely to have normal TCD results. Transfusion resulted in iron overload and alloimmunization, but no infection. The study provides new information on acceptance rates and long-term effects of transfusion. Persistent TCD elevation signals ongoing stroke risk. Reduction in TCD results over time without transfusion is observed in some patients and requires further study.
Figures
Figure 1.
Treatment history. (A) Transfusion group. (B) Standard care group. Dates indicate study time points: February 1995 being the start of STOP Trial and September 1997, the end; January 1998 is the start of post-trial followup, and June 2000, its end. Numbers in parentheses represent the number of patients.
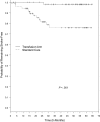
Figure 2.
Kaplan-Meier estimates of the probability of remaining stroke-free among patients receiving transfusion and patients on standard care. The P value was calculated by log-rank test. Tick marks indicate the lengths of observation of patients who did not have a stroke. Four patients were not included in this analysis: 1 patient from the standard care group who experienced intracerebral hematoma as noted on baseline MRI, and 3 patients who developed stroke after change in treatment during the posttrial follow-up (2 from the standard care and 1 from the transfusion group).
Similar articles
- Transcranial Doppler velocity and brain MRI/MRA changes in children with sickle cell anemia on chronic transfusions to prevent primary stroke.
Sheehan VA, Hansbury EN, Smeltzer MP, Fortner G, McCarville MB, Aygun B. Sheehan VA, et al. Pediatr Blood Cancer. 2013 Sep;60(9):1499-502. doi: 10.1002/pbc.24569. Epub 2013 Apr 26. Pediatr Blood Cancer. 2013. PMID: 23625812 Clinical Trial. - Blood transfusion for preventing primary and secondary stroke in people with sickle cell disease.
Wang WC, Dwan K. Wang WC, et al. Cochrane Database Syst Rev. 2013 Nov 14;(11):CD003146. doi: 10.1002/14651858.CD003146.pub2. Cochrane Database Syst Rev. 2013. PMID: 24226646 Free PMC article. Updated. Review. - Effect of transfusion therapy on transcranial Doppler ultrasonography velocities in children with sickle cell disease.
Kwiatkowski JL, Yim E, Miller S, Adams RJ; STOP 2 Study Investigators. Kwiatkowski JL, et al. Pediatr Blood Cancer. 2011 May;56(5):777-82. doi: 10.1002/pbc.22951. Epub 2010 Dec 23. Pediatr Blood Cancer. 2011. PMID: 21370410 Free PMC article. - The clinical effectiveness and cost-effectiveness of primary stroke prevention in children with sickle cell disease: a systematic review and economic evaluation.
Cherry MG, Greenhalgh J, Osipenko L, Venkatachalam M, Boland A, Dundar Y, Marsh K, Dickson R, Rees DC. Cherry MG, et al. Health Technol Assess. 2012;16(43):1-129. doi: 10.3310/hta16430. Health Technol Assess. 2012. PMID: 23140544 Free PMC article. Review. - Design of the DREPAGREFFE trial: A prospective controlled multicenter study evaluating the benefit of genoidentical hematopoietic stem cell transplantation over chronic transfusion in sickle cell anemia children detected to be at risk of stroke by transcranial Doppler (NCT 01340404).
Chevret S, Verlhac S, Ducros-Miralles E, Dalle JH, de Latour RP, de Montalembert M, Benkerrou M, Pondarré C, Thuret I, Guitton C, Lesprit E, Etienne-Julan M, Elana G, Vannier JP, Lutz P, Neven B, Galambrun C, Paillard C, Runel C, Jubert C, Arnaud C, Kamdem A, Brousse V, Missud F, Petras M, Doumdo-Divialle L, Berger C, Fréard F, Taieb O, Drain E, Elmaleh M, Vasile M, Khelif Y, Bernaudin M, Chadebech P, Pirenne F, Socié G, Bernaudin F. Chevret S, et al. Contemp Clin Trials. 2017 Nov;62:91-104. doi: 10.1016/j.cct.2017.08.008. Epub 2017 Aug 15. Contemp Clin Trials. 2017. PMID: 28821470 Clinical Trial.
Cited by
- Red blood cell alloimmunization in sickle cell disease: prevalence in 2010.
Miller ST, Kim HY, Weiner DL, Wager CG, Gallagher D, Styles LA, Dampier CD, Roseff SD; Investigators of the Sickle Cell Disease Clinical Research Network (SCDCRN). Miller ST, et al. Transfusion. 2013 Apr;53(4):704-9. doi: 10.1111/j.1537-2995.2012.03796.x. Epub 2012 Jul 13. Transfusion. 2013. PMID: 22804353 Free PMC article. Clinical Trial. - Myocardial ischaemia in sickle cell anaemia: evaluation using a new scoring system.
Bode-Thomas F, Hyacinth HI, Ogunkunle O, Omotoso A. Bode-Thomas F, et al. Ann Trop Paediatr. 2011;31(1):67-74. doi: 10.1179/1465328110Y.0000000006. Ann Trop Paediatr. 2011. PMID: 21262112 Free PMC article. - Hemoglobin A clearance in children with sickle cell anemia on chronic transfusion therapy.
Yee MEM, Josephson CD, Winkler AM, Webb J, Luban NLC, Leong T, Stowell SR, Roback JD, Fasano RM. Yee MEM, et al. Transfusion. 2018 Jun;58(6):1363-1371. doi: 10.1111/trf.14610. Epub 2018 Apr 17. Transfusion. 2018. PMID: 29664198 Free PMC article. - Prevalence and risk factors for venous thromboembolism in children with sickle cell disease: an administrative database study.
Kumar R, Stanek J, Creary S, Dunn A, O'Brien SH. Kumar R, et al. Blood Adv. 2018 Feb 13;2(3):285-291. doi: 10.1182/bloodadvances.2017012336. Blood Adv. 2018. PMID: 29431623 Free PMC article. - Blood transfusion for preventing primary and secondary stroke in people with sickle cell disease.
Estcourt LJ, Fortin PM, Hopewell S, Trivella M, Wang WC. Estcourt LJ, et al. Cochrane Database Syst Rev. 2017 Jan 17;1(1):CD003146. doi: 10.1002/14651858.CD003146.pub3. Cochrane Database Syst Rev. 2017. PMID: 28094851 Free PMC article. Updated. Review.
References
- Ohene-Frempong K, Weiner S, Sleeper L, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91: 288-294. - PubMed
- Adams R, McKie V, Nichols F, et al. The use of transcranial ultrasonography to predict stroke in sickle cell disease. N Engl J Med. 1992;326: 605-610. - PubMed
- Adams RJ, McKie VC, Carl EM, et al. Long-term stroke risk in children with sickle cell disease screened with transcranial Doppler. Ann Neurol. 1997;42: 699-704. - PubMed
- Adams R, McKie V, Hsu L, et al. Prevention of a first stroke by transfusion in children with abnormal results of transcranial Doppler ultrasonography. N Engl J Med. 1998;339: 5-11. - PubMed
- NHLBI Clinical Alert: Periodic transfusions lower stroke risk in children with sickle cell anemia (http://www.nlm.nih.gov/databases/alerts/sickle97.html). Bethesda, MD: National Institutes of Health, 1997. Accessed August 6, 2005.