Hemin-dependent modulation of the lipid A structure of Porphyromonas gingivalis lipopolysaccharide - PubMed (original) (raw)
Hemin-dependent modulation of the lipid A structure of Porphyromonas gingivalis lipopolysaccharide
Montaser N Al-Qutub et al. Infect Immun. 2006 Aug.
Abstract
Porphyromonas gingivalis is a periopathogen strongly associated with the development of adult-type periodontitis. Both the virulence characteristics of periopathogens and host-related factors are believed to contribute to periodontitis. P. gingivalis lipopolysaccharide (LPS) displays a significant amount of lipid A structural heterogeneity, containing both penta- and tetra-acylated lipid A structures. However, little is known concerning how the lipid A structural content of P. gingivalis is regulated. Alterations in the lipid A content may facilitate the ability of P. gingivalis to modulate the innate host response to this bacterium. In this report, it is shown that the concentration of hemin in the growth medium significantly modulates the lipopolysaccharide lipid A structural content of P. gingivalis. Hemin is a key microenvironmental component of gingival cervicular fluid which is believed to vary depending upon the state of vascular ulceration. At low hemin concentrations, one major penta-acylated lipid A structure was found, whereas at high concentrations of hemin, multiple tetra- and penta-acylated lipid A structures were observed. Hemin concentrations, not iron acquisition, were responsible for the alterations in the lipid A structural content. The modifications of the lipid A structural content were independent of the LPS extraction procedure and occurred in a variety of laboratory strains as well as a freshly obtained clinical isolate. The known hemin binding proteins Kgp and HmuR contributed to the lipid A modulation sensing mechanism. To the best of our knowledge, this is the first report that hemin, a clinically relevant microenvironmental component for P. gingivalis, can modulate the lipid A structure found in a bacterium. Since tetra- and penta-acylated P. gingivalis lipid A structures have opposing effects on Toll-like receptor 4 activation, the alteration of the lipid A structural content may have significant effects on the host response to this bacterium.
Figures
FIG. 1.
Structures of previously characterized P. gingivalis lipid A mass ions. P. gingivalis LPS contains several different lipid A structures that differ in their numbers of phosphate groups and fatty acids. In this figure, the MALDI-TOF lipid A peaks that have been characterized with respect to their structure are presented. (A) Structure determined by Ogawa (16); (B to E) structures elucidated by Kumada et al. (13).
FIG. 2.
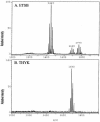
Effect of in vitro culture medium on P. gingivalis lipid A structural content. (A) P. gingivalis strain 33277 incubated in ETSB medium; (B) the same strain incubated in TYHK medium (see the text for details). P. gingivalis LPS was obtained by the Tri-Reagent procedure (30), and lipid A was cleaved and separated from the LPS as described by Caroff et al. (3). MALDI-TOF analysis was performed as previously described (11). All values given are average masses rounded to the nearest whole numbers for singly charged deprotonated molecules. Note that the in vitro culture medium significantly affected the lipid A structural content.
FIG. 3.
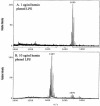
Effect of hemin concentration on lipid A content of P. gingivalis strain 33277. P. gingivalis strain 33277 was incubated in TYHK medium with either 1 (A) or 10 (B) μg/ml hemin. P. gingivalis LPS was obtained by the Tri-Reagent procedure (28), and lipid A was cleaved and separated from the LPS as described by Caroff et al. (3). MALDI-TOF analysis was performed as previously described (11). All values given are average masses rounded to the nearest whole numbers for singly charged deprotonated molecules. Note that at 1 μg/ml hemin, the lipid A content was similar to that for growth in TYHK medium, whereas at 10 μg/ml hemin, the lipid A content resembled that found in ETSB medium (see Fig. 2 for comparison).
FIG. 4.
Effect of phenol-water LPS isolation procedure on lipid A content of P. gingivalis strain 33277. P. gingivalis strain 33277 was incubated in TYHK medium with either 1 (A) or 10 (B) μg/ml hemin. P. gingivalis LPS was obtained by the phenol-water isolation procedure (30), and lipid A was cleaved and separated from the LPS as described by Caroff et al. (3). MALDI-TOF analysis was performed as previously described (11). All values given are average masses rounded to the nearest whole numbers for singly charged deprotonated molecules. Note that the phenol-water isolation procedure failed to effectively extract lipid A structures centered at m/z 1,770 (see Fig. 3, bottom panel, for comparison).
FIG. 5.
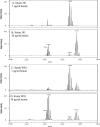
Effects of various FeCl2 concentrations on lipid A content of P. gingivalis strain 33277. P. gingivalis strain 33277 was incubated in TYHK medium containing no hemin and either 13.5 (A), 54 (B), or 337.5 (C) μg/ml FeCl2. P. gingivalis LPS was obtained by the Tri-Reagent procedure (30), and lipid A was cleaved and separated from the LPS as described by Caroff et al. (3). MALDI-TOF analysis was performed as previously described (11). All values given are average masses rounded to the nearest whole numbers for singly charged deprotonated molecules. Note that the FeCl2 concentration did not significantly alter the lipid A content.
FIG. 6.
Effect of hemin concentration on lipid A contents of different P. gingivalis strains. P. gingivalis laboratory strains 381 (A and B) and W83 (C and D) and a freshly obtained P. gingivalis clinical isolate (E and F) were incubated in TYHK medium with either 1 (A, C, and E) or 10 (B, D, and F) μg/ml hemin. P. gingivalis LPS was obtained by the Tri-Reagent procedure (30), and lipid A was cleaved and separated from the LPS as described by Caroff et al. (3). MALDI-TOF analysis was performed as previously described (11). All values given are average masses rounded to the nearest whole numbers for singly charged deprotonated molecules. Note that the concentration of hemin affected the lipid A contents of several different strains in a similar fashion.
FIG. 6.
Effect of hemin concentration on lipid A contents of different P. gingivalis strains. P. gingivalis laboratory strains 381 (A and B) and W83 (C and D) and a freshly obtained P. gingivalis clinical isolate (E and F) were incubated in TYHK medium with either 1 (A, C, and E) or 10 (B, D, and F) μg/ml hemin. P. gingivalis LPS was obtained by the Tri-Reagent procedure (30), and lipid A was cleaved and separated from the LPS as described by Caroff et al. (3). MALDI-TOF analysis was performed as previously described (11). All values given are average masses rounded to the nearest whole numbers for singly charged deprotonated molecules. Note that the concentration of hemin affected the lipid A contents of several different strains in a similar fashion.
FIG.7.
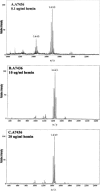
Effect of hemin concentration on lipid A content of a P. gingivalis hemin acquisition mutant. P. gingivalis strain A7436 (A, B, and C) and its isogenic Kgp/HmuR double mutant strain WS15 (D, E, and F) were incubated in TYHK medium with various concentrations of hemin, as indicated in each panel. P. gingivalis LPS was obtained by the Tri-Reagent procedure (30), and lipid A was cleaved and separated from the LPS as described by Caroff et al. (3). MALDI-TOF analysis was performed as previously described (11). All values given are average masses rounded to the nearest whole numbers for singly charged deprotonated molecules. Note that strain WS15 required a higher concentration of hemin to alter its lipid A content.
FIG.7.
Effect of hemin concentration on lipid A content of a P. gingivalis hemin acquisition mutant. P. gingivalis strain A7436 (A, B, and C) and its isogenic Kgp/HmuR double mutant strain WS15 (D, E, and F) were incubated in TYHK medium with various concentrations of hemin, as indicated in each panel. P. gingivalis LPS was obtained by the Tri-Reagent procedure (30), and lipid A was cleaved and separated from the LPS as described by Caroff et al. (3). MALDI-TOF analysis was performed as previously described (11). All values given are average masses rounded to the nearest whole numbers for singly charged deprotonated molecules. Note that strain WS15 required a higher concentration of hemin to alter its lipid A content.
Similar articles
- Porphyromonas gingivalis lipopolysaccharide lipid A heterogeneity: differential activities of tetra- and penta-acylated lipid A structures on E-selectin expression and TLR4 recognition.
Reife RA, Coats SR, Al-Qutub M, Dixon DM, Braham PA, Billharz RJ, Howald WN, Darveau RP. Reife RA, et al. Cell Microbiol. 2006 May;8(5):857-68. doi: 10.1111/j.1462-5822.2005.00672.x. Cell Microbiol. 2006. PMID: 16611234 - Hemin binding by Porphyromonas gingivalis strains is dependent on the presence of A-LPS.
Rangarajan M, Aduse-Opoku J, Paramonov NA, Hashim A, Curtis MA. Rangarajan M, et al. Mol Oral Microbiol. 2017 Oct;32(5):365-374. doi: 10.1111/omi.12178. Epub 2017 Mar 9. Mol Oral Microbiol. 2017. PMID: 28107612 Free PMC article. - Regulation of hemin and iron transport in Porphyromonas gingivalis.
Genco CA. Genco CA. Adv Dent Res. 1995 Feb;9(1):41-7. doi: 10.1177/08959374950090010801. Adv Dent Res. 1995. PMID: 7669213 Review. - Chemical structure and immunobiological activity of Porphyromonas gingivalis lipid A.
Ogawa T, Asai Y, Makimura Y, Tamai R. Ogawa T, et al. Front Biosci. 2007 May 1;12:3795-812. doi: 10.2741/2353. Front Biosci. 2007. PMID: 17485340 Review.
Cited by
- Subgingival Plaque in Periodontal Health Antagonizes at Toll-Like Receptor 4 and Inhibits E-Selectin Expression on Endothelial Cells.
To TT, Gümüş P, Nizam N, Buduneli N, Darveau RP. To TT, et al. Infect Immun. 2015 Oct 19;84(1):120-6. doi: 10.1128/IAI.00693-15. Print 2016 Jan. Infect Immun. 2015. PMID: 26483407 Free PMC article. - Exploring the Association between Alzheimer's Disease, Oral Health, Microbial Endocrinology and Nutrition.
Harding A, Gonder U, Robinson SJ, Crean S, Singhrao SK. Harding A, et al. Front Aging Neurosci. 2017 Dec 1;9:398. doi: 10.3389/fnagi.2017.00398. eCollection 2017. Front Aging Neurosci. 2017. PMID: 29249963 Free PMC article. Review. - Comparative analysis of Porphyromonas gingivalis A7436 and ATCC 33277 strains reveals differences in the expression of heme acquisition systems.
Śmiga M, Ślęzak P, Olczak T. Śmiga M, et al. Microbiol Spectr. 2024 Mar 5;12(3):e0286523. doi: 10.1128/spectrum.02865-23. Epub 2024 Jan 30. Microbiol Spectr. 2024. PMID: 38289063 Free PMC article. - Porphyromonas gingivalis lipids inhibit osteoblastic differentiation and function.
Wang YH, Jiang J, Zhu Q, AlAnezi AZ, Clark RB, Jiang X, Rowe DW, Nichols FC. Wang YH, et al. Infect Immun. 2010 Sep;78(9):3726-35. doi: 10.1128/IAI.00225-10. Epub 2010 Jun 28. Infect Immun. 2010. PMID: 20584977 Free PMC article. - Human Toll-like receptor 4 responses to P. gingivalis are regulated by lipid A 1- and 4'-phosphatase activities.
Coats SR, Jones JW, Do CT, Braham PH, Bainbridge BW, To TT, Goodlett DR, Ernst RK, Darveau RP. Coats SR, et al. Cell Microbiol. 2009 Nov;11(11):1587-99. doi: 10.1111/j.1462-5822.2009.01349.x. Epub 2009 Jun 13. Cell Microbiol. 2009. PMID: 19552698 Free PMC article.
References
- Bainbridge, B. W., S. R. Coats, and R. P. Darveau. 2002. Porphyromonas gingivalis lipopolysaccharide displays functionally diverse interactions with the innate host defense system. Ann. Periodontol. 7:1-9. - PubMed
- Beutler, B. 2000. Tlr4: central component of the sole mammalian LPS sensor. Curr. Opin. Immunol. 12:20-26. - PubMed
- Caroff, M., A. Tacken, and L. Szabó. 1988. Detergent-accelerated hydrolysis of bacterial endotoxins and determination of the anomeric configuration of the glycosyl phosphate present in the “isolated lipid A” fragment of the Bordetella pertussis endotoxin. Carbohydr. Res. 175:273-282. - PubMed
- Champagne, C. M., S. C. Holt, T. E. Van Dyke, B. J. Gordon, and L. Shapira. 1996. Lipopolysaccharide isolated from Porphyromonas gingivalis grown in hemin-limited chemostat conditions has a reduced capacity for human neutrophil priming. Oral Microbiol. Immunol. 11:319-325. - PubMed
- Coats, S. R., T. T. Pham, B. W. Bainbridge, R. A. Reife, and R. P. Darveau. 2005. MD-2 mediates the ability of tetra-acylated and penta-acylated lipopolysaccharides to antagonize Escherichia coli lipopolysaccharide at the TLR4 signaling complex. J. Immunol. 175:4490-4498. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases