Maturation of intracellular Escherichia coli communities requires SurA - PubMed (original) (raw)
Maturation of intracellular Escherichia coli communities requires SurA
Sheryl S Justice et al. Infect Immun. 2006 Aug.
Abstract
Escherichia coli is the most common cause of community-acquired urinary tract infection (UTI). During murine cystitis, uropathogenic E. coli (UPEC) utilizes type 1 pili to bind and invade superficial bladder epithelial cells. UPEC then replicates within to form intracellular bacterial communities (IBCs), a process whose genetic determinants are as yet undefined. In this study, we investigated the role of SurA in the UPEC pathogenic cascade. SurA is a periplasmic prolyl isomerase/chaperone that facilitates outer membrane protein biogenesis and pilus assembly in E. coli. Invasion into bladder epithelial cells was disproportionately reduced when surA was genetically disrupted in the UPEC strain UTI89, demonstrating that binding alone is not sufficient for invasion. In a murine cystitis model, UTI89 surA::kan was unable to persist in the urinary tract. Complementation of UTI89 surA::kan with a plasmid (pDH15) containing surA under the control of an arabinose-inducible promoter restored in vivo binding and invasion events. However, the absence of arabinose within the mouse bladder resulted in depletion of SurA after invasion of the bacteria into the superficial epithelial cells. Under these conditions, invasion by UTI89/pDH15 surA::kan was normal, but in contrast to UTI89, UTI89/pDH15 surA::kan formed intracellular collections that contained fewer bacteria, were loosely organized, and lacked the normal transition to a densely packed, coccoid morphology. Our data argue that SurA is required within bladder epithelial cells for UPEC to undergo the morphological changes that underlie IBC maturation and completion of the UTI pathogenic cascade.
Figures
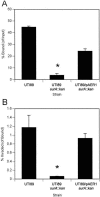
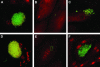
FIG. 1.
Mutation in surA confers a disproportionate effect on bacterial invasion in vitro. Monolayers of cultured 5637 bladder epithelial cells were infected with wild-type UTI89, UTI89 surA::kan, or UTI89 surA::kan complemented with plasmid pAER1, and bacteria bound (as a proportion of input bacteria) and invaded (as a proportion of bound bacteria) were quantified. The surA mutant demonstrates reduced binding (A) and an additive defect in invasion capacity (B) (*, P < 0.005 versus wild type). Experiments were repeated at least three times with similar results.
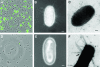
FIG. 2.
Microscopy of surA mutant UPEC. Immunofluorescence of an overnight static culture of UTI89 demonstrates type 1 piliation (A), while UTI89 surA::kan is not labeled with anti-FimHA antibody (D). Negative-stain EM reveals that, in contrast to wild-type UTI89 (B), the majority of UTI89 surA::kan bacteria are nonpiliated (E). Immunogold EM with anti-FimHA antibody identifies type 1 pili on the surface of UTI89 (C) but does not consistently label the thicker, bundled fibers (arrow) expressed by a minority of surA mutant bacteria (F). Scale bars, 200 nm.
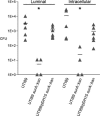
FIG. 3.
In vivo invasion is intact in the UTI89 surA mutant complemented with pDH15 in an ex vivo gentamicin protection assay. C3H/HeN female mice were transurethrally inoculated with wild-type UTI89, UTI89 surA::kan, or UTI89/pDH15 surA::kan, and bladders were harvested after 1 h and processed as described in Materials and Methods. Luminal bacteria and invaded bacteria (intracellular) were quantified by serial dilution and plating. UTI89 surA::kan persists poorly in the lumen over 1 h of infection and invades poorly compared to wild-type UTI89 (*, P < 0.005); complementation with pDH15 restores early luminal colonization and invasion to wild-type levels. Experiments were repeated at least three times with similar results.
FIG. 4.
The UPEC surA mutant is deficient in establishing murine cystitis. Mice were infected with the wild type or UTI89 surA::kan and the bladders harvested for titers at the indicated time points. Wild-type UTI89 persists at 106 CFU/bladder during the first 48 h of infection, while UTI89 surA::kan titers fall off and are nearly undetectable at 2 weeks after infection. Titers of UTI89 surA::kan are significantly lower than those of wild-type UTI89 at all time points (*, P < 0.0001; **, P < 0.001). Horizontal bars indicate mean titers. Experiments were repeated at least three times.
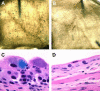
FIG. 5.
The UPEC surA mutant fails to establish IBCs. C3H/HeN mice were inoculated with wild-type UTI89, UTI89 surA::kan, or UTI89/pDH15 surA::kan; bladders were harvested at 6 h after infection and exposed to an X-Gal-containing substrate (see Materials and Methods). In whole mount, wild-type IBCs are visible as dark blue spots on the bladder surface (A), while UTI89 surA::kan (B) infection yields no such staining. By light microscopy, UTI89-infected bladders contain IBCs; panel C includes an example in which deparaffinization incompletely removed the lacZ stain from an IBC. Bladders infected with UTI89 surA::kan (D) or UTI89/pDH15 surA::kan (not shown) contain no developed IBCs. Experiments were repeated at least three times.
FIG. 6.
SurA is required for IBC maturation. UTI89/pcomGFP, UTI89/pcomGFP surA::kan, and UTI89/pDH15 surA::kan were used to inoculate C3H/HeN female mice, and the bladders were viewed in whole mount by confocal microscopy at 6 h (top row) and 16 h (bottom row). UTI89 forms early IBCs at 6 h (A) and matures into tightly packed coccoid IBCs by 16 h (D), while the UTI89 surA mutant fails to establish intracellular growth (B). At 16 h, a single collection of ∼20 intracellular bacteria was seen in one of >20 bladders infected with UTI89 surA::kan across several replicated experiments (E). Complementation of type 1 piliation in vitro and binding and invasion in vivo via the plasmid pDH15 permit invasion and modest intracellular replication by 6 h (C), but UTI89/pDH15 surA::kan IBCs do not mature properly by 16 h (F), demonstrating lower density, looser organization, and persistent rod-shaped bacterial morphology compared to wild-type IBCs.
FIG. 7.
Model depicting the contributions of SurA to steps in the UPEC pathogenic cascade. SurA supports binding and invasion of bladder epithelial cells through type 1 pilus assembly. In addition, other SurA substrates may potentiate invasion. SurA activity is required for intracellular growth of UPEC and for maturation of IBCs. Finally, suppression of bladder epithelial cytokines in vitro also requires SurA (15), which may impact bacterial persistence (dashed line).
Similar articles
- Components of SurA required for outer membrane biogenesis in uropathogenic Escherichia coli.
Watts KM, Hunstad DA. Watts KM, et al. PLoS One. 2008 Oct 6;3(10):e3359. doi: 10.1371/journal.pone.0003359. PLoS One. 2008. PMID: 18836534 Free PMC article. - Utilization of an intracellular bacterial community pathway in Klebsiella pneumoniae urinary tract infection and the effects of FimK on type 1 pilus expression.
Rosen DA, Pinkner JS, Jones JM, Walker JN, Clegg S, Hultgren SJ. Rosen DA, et al. Infect Immun. 2008 Jul;76(7):3337-45. doi: 10.1128/IAI.00090-08. Epub 2008 Apr 14. Infect Immun. 2008. PMID: 18411285 Free PMC article. - Development of intracellular bacterial communities of uropathogenic Escherichia coli depends on type 1 pili.
Wright KJ, Seed PC, Hultgren SJ. Wright KJ, et al. Cell Microbiol. 2007 Sep;9(9):2230-41. doi: 10.1111/j.1462-5822.2007.00952.x. Epub 2007 May 8. Cell Microbiol. 2007. PMID: 17490405 - The role of SurA factor in outer membrane protein transport and virulence.
Behrens-Kneip S. Behrens-Kneip S. Int J Med Microbiol. 2010 Nov;300(7):421-8. doi: 10.1016/j.ijmm.2010.04.012. Epub 2010 May 6. Int J Med Microbiol. 2010. PMID: 20447864 Review. - Covert operations of uropathogenic Escherichia coli within the urinary tract.
Bower JM, Eto DS, Mulvey MA. Bower JM, et al. Traffic. 2005 Jan;6(1):18-31. doi: 10.1111/j.1600-0854.2004.00251.x. Traffic. 2005. PMID: 15569242 Free PMC article. Review.
Cited by
- Polysaccharide capsule and sialic acid-mediated regulation promote biofilm-like intracellular bacterial communities during cystitis.
Anderson GG, Goller CC, Justice S, Hultgren SJ, Seed PC. Anderson GG, et al. Infect Immun. 2010 Mar;78(3):963-75. doi: 10.1128/IAI.00925-09. Epub 2010 Jan 19. Infect Immun. 2010. PMID: 20086090 Free PMC article. - Urothelial cultures support intracellular bacterial community formation by uropathogenic Escherichia coli.
Berry RE, Klumpp DJ, Schaeffer AJ. Berry RE, et al. Infect Immun. 2009 Jul;77(7):2762-72. doi: 10.1128/IAI.00323-09. Epub 2009 May 18. Infect Immun. 2009. PMID: 19451249 Free PMC article. - Microbial peptidyl-prolyl cis/trans isomerases (PPIases): virulence factors and potential alternative drug targets.
Ünal CM, Steinert M. Ünal CM, et al. Microbiol Mol Biol Rev. 2014 Sep;78(3):544-71. doi: 10.1128/MMBR.00015-14. Microbiol Mol Biol Rev. 2014. PMID: 25184565 Free PMC article. Review. - Catheterization of mice triggers resurgent urinary tract infection seeded by a bladder reservoir of Acinetobacter baumannii.
Hazen JE, Di Venanzio G, Hultgren SJ, Feldman MF. Hazen JE, et al. Sci Transl Med. 2023 Jan 11;15(678):eabn8134. doi: 10.1126/scitranslmed.abn8134. Epub 2023 Jan 11. Sci Transl Med. 2023. PMID: 36630484 Free PMC article. - Virulence Mechanisms of Common Uropathogens and Their Intracellular Localisation within Urothelial Cells.
Ognenovska S, Mukerjee C, Sanderson-Smith M, Moore KH, Mansfield KJ. Ognenovska S, et al. Pathogens. 2022 Aug 17;11(8):926. doi: 10.3390/pathogens11080926. Pathogens. 2022. PMID: 36015046 Free PMC article.
References
- Anderson, G. G., J. J. Palermo, J. D. Schilling, R. Roth, J. Heuser, and S. J. Hultgren. 2003. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301:105-107. - PubMed
- Bitto, E., and D. B. McKay. 2003. The periplasmic molecular chaperone protein SurA binds a peptide motif that is characteristic of integral outer membrane proteins. J. Biol. Chem. 278:49316-49322. - PubMed
- Chen, S. L., C. S. Hung, J. Xu, C. S. Reigstad, V. Magrini, A. Sabo, D. Blasiar, T. Bieri, R. R. Meyer, P. Ozersky, J. R. Armstrong, R. S. Fulton, J. P. Latreille, J. Spieth, T. M. Hooton, E. R. Mardis, S. J. Hultgren, and J. I. Gordon. 2006. Identification of genes subject to positive selection in uropathogenic strains of Escherichia coli: a comparative genomics approach. Proc. Natl. Acad. Sci. USA 103:5977-5982. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-DK51406/DK/NIDDK NIH HHS/United States
- R01 AI029549/AI/NIAID NIH HHS/United States
- P50-DK64540/DK/NIDDK NIH HHS/United States
- F32 DK010168/DK/NIDDK NIH HHS/United States
- P50 DK064540/DK/NIDDK NIH HHS/United States
- K08 DK067894/DK/NIDDK NIH HHS/United States
- R01-AI29549/AI/NIAID NIH HHS/United States
- R01 DK051406/DK/NIDDK NIH HHS/United States
- F32-DK10168/DK/NIDDK NIH HHS/United States
- K08-DK067894/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical