Identification and characterization of a lysophosphatidylcholine acyltransferase in alveolar type II cells - PubMed (original) (raw)
Identification and characterization of a lysophosphatidylcholine acyltransferase in alveolar type II cells
Xueni Chen et al. Proc Natl Acad Sci U S A. 2006.
Abstract
Pulmonary surfactant is a complex of lipids and proteins produced and secreted by alveolar type II cells that provides the low surface tension at the air-liquid interface. The phospholipid most responsible for providing the low surface tension in the lung is dipalmitoylphosphatidylcholine. Dipalmitoylphosphatidylcholine is synthesized in large part by phosphatidylcholine (PC) remodeling, and a lysophosphatidylcholine (lysoPC) acyltransferase is thought to play a critical role in its synthesis. However, this acyltransferase has not yet been identified. We have cloned full-length rat and mouse cDNAs coding for a lysoPC acyltransferase (LPCAT). LPCAT encodes a 535-aa protein of approximately 59 kDa that contains a transmembrane domain and a putative acyltransferase domain. When transfected into COS-7 cells and HEK293 cells, LPCAT significantly increased lysoPC acyltransferase activity. LPCAT preferred lysoPC as a substrate over lysoPA, lysoPI, lysoPS, lysoPE, or lysoPG and prefers palmitoyl-CoA to oleoyl-CoA as the acyl donor. This LPCAT was preferentially expressed in the lung, specifically within alveolar type II cells. Expression in the fetal lung and in rat type II cells correlated with the expression of the surfactant proteins. LPCAT expression in fetal lung explants was sensitive to dexamethasone and FGFs. KGF was a potent stimulator of LPCAT expression in cultured adult type II cells. We hypothesize that LPCAT plays a critical role in regulating surfactant phospholipid biosynthesis and suggest that understanding the regulation of LPCAT will offer important insight into surfactant phospholipid biosynthesis.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
Fig. 1.
LPCAT protein sequence. The structure of LPCAT has a transmembrane domain, a domain for glycerolipid acyltransferase activity, and two EF hand (EFH) domains for potential calcium binding sites. The domain for glycerolipid acyltransferase activity is highlighted, and the critical H(X)4D signature is underlined.
Fig. 2.
LysoPC acyltransferase activity. (A) Expression of rat LPCAT in mammalian cells. Forty-eight hours after transfection, cells were processed for Western blot using an anti-V5 antibody. Lane 1, wild-type COS-7 cells; lane 2, COS cells transfected with the empty vector; lane 3, COS-7 cells transfected with LPCAT; lane 4, wild-type HEK293 cells; lane 5, HEK293 cells transfected with the empty vector; lane 6, HEK293 cells transfected with LPCAT. (B) The acyltransferase activities of the recombinant LPCAT expressed in HEK293 cells with different lysophospholipid substrates. The acyltransferase assays were conducted by incubating 20 μM [1-14C]palmitoyl-CoA with each of the lysophospholipids (150 μM) in the presence of 20 μg of cell homogenates from either wild-type cells (293-W), cells transfected with control vector (293-C), or with LPCAT (293-LPCAT). All enzyme activity data are derived from at least three independent experiments and are shown as mean ± SE. ∗, a significant difference (P < 0.001) from the vector control. (C) The preference of palmitoyl-CoA over oleoyl-CoA on lysoPC acyltransferase activity of recombinant (R) LPCAT expressed in HEK293 cells compared with C (control, empty vector). The enzyme activity was performed by incubating 150 μM lysoPC with 20 μM each [14C]acyl-CoA for 0, 5, 10, and 15 min. The data are derived from at least three independent experiments and are shown as mean ± SE.
Fig. 3.
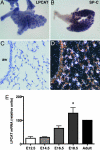
LPCAT expression in the developing and adult lung. Lungs were harvested from mouse embryos on days E13.5, E15.5, and E18.5, as well as from adults. Whole mount ISH on day E13.5 (A) showed that LPCAT was expressed intensely in the distal epithelial tips but not in proximal major airways. This expression pattern was identical to that seen for SP-C (B). Lungs hybridized with a sense LPCAT probe gave no signal (data not shown). Sections of E15.5 and E18.5 lung hybridized with LPCAT probe showed specific localization to distal acini, whereas large airways were negative. In the adult lung (C and D), LPCAT expression was highest in epithelial cells of the alveolar wall and was not present in airway (aw) epithelium. (E) Real-time PCR was used to determine LPCAT mRNA expression levels in the developing lung normalized to β-actin and compared with the levels in adult lung. The results are means ± SEM from three independent determinations at each gestational age. ∗, significantly (P < 0.01) increased compared with days E12.5, E14.5, and E16.5.
Fig. 4.
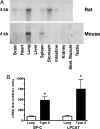
LPCAT expression in adult organs. (A) Northern blots were prepared by using 10 μg of total RNA from various rat and mouse organs, then hybridized with radiolabeled mouse LPCAT cDNA. Expression was most abundant in the lung for both rat and mouse. The rat also showed significant expression in the stomach, along with faint expression in the brain, spleen, and intestine. In the mouse, faint expression was also detected in the spleen. (B) Real-time PCR was used to compare LPCAT and SP-C mRNA content in adult mouse lungs and freshly isolated mouse alveolar type II cells. Normalized to β-actin, LPCAT content was enriched 7.4-fold in type II cells, and SP-C content was enriched 4.8-fold. The results are means ± SEM from four independent experiments. ∗, significantly greater (P < 0.05) than whole lung.
Fig. 5.
LPCAT expression is regulated by glucocorticoids and FGFs in the developing lung. E16.5 lung explants were cultured for 1–2 days in medium containing 10−7 M Dex or 10 μM SU5402; 0.1% ethanol (EtOH) or 0.1% DMSO served as respective vehicle controls. LPCAT mRNA content, normalized to β-actin, was determined by real-time PCR. The results are means ± SE from three independent experiments. Compared with time 0 tissue, LPCAT expression was significantly increased (P < 0.001) in the presence of EtOH, DMSO, or no additions (+0) at both 24 and 48 h of culture. Compared with EtOH, the addition of Dex caused a significant (P < 0.001) increase in LPCAT expression at both 24 and 48 h. In contrast, abrogation of FGF signaling with SU5402 significantly (P < 0.01) inhibited the increase in LPCAT expression seen in DMSO treated cultures. ∗, significantly different from vehicle control; †, significantly different from the same treatment at 24 h.
Fig. 6.
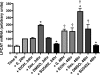
Regulation of LPCAT in type II cells. (A) mRNA levels of LPCAT are increased by KGF in rat alveolar type II cells. Type II cells were cultured for 5 days with and without KGF (10 ng/ml). The mRNA levels were determined by real-time PCR, as described in Materials and Methods. The results were normalized with cyclophilin B and expressed as a fold change in comparison with the cultures without KGF. The results are the means ± SE for five experiments. ∗, a significant increase (P < 0.01) from the control culture. KGF increased LPCAT levels (R1), whereas the expression of the other putative acyltransferases (R2–R4) was unchanged. The mRNA for R3 was below the level of detection (ND). (B_–_D) Rat alveolar type II cells were cultured in plastic wells with 10% FBS. Cells were harvested on the indicated days. The mRNA levels of LPCAT, SP-A (type II cell marker), and T1α (type I cell marker) were examined by real-time PCR and normalized to GAPDH. (B) The mRNA expression of SP-A decreased during the in vitro transition from type II cells to type I-like cells. (C) The expression of LPCAT decreased in parallel with SP-A, whereas the expression of T1α increased as expected under these culture conditions (D). The results are the means ± SE for three experiments. ∗, a significant difference (P < 0.05) from the control culture.
Similar articles
- Cloning and characterization of mouse lung-type acyl-CoA:lysophosphatidylcholine acyltransferase 1 (LPCAT1). Expression in alveolar type II cells and possible involvement in surfactant production.
Nakanishi H, Shindou H, Hishikawa D, Harayama T, Ogasawara R, Suwabe A, Taguchi R, Shimizu T. Nakanishi H, et al. J Biol Chem. 2006 Jul 21;281(29):20140-7. doi: 10.1074/jbc.M600225200. Epub 2006 May 16. J Biol Chem. 2006. PMID: 16704971 - Molecular Characterization of Two Lysophospholipid:acyl-CoA Acyltransferases Belonging to the MBOAT Family in Nicotiana benthamiana.
Zhang D, Jasieniecka-Gazarkiewicz K, Wan X, Luo L, Zhang Y, Banas A, Jiang M, Gong Y. Zhang D, et al. PLoS One. 2015 Dec 18;10(12):e0144653. doi: 10.1371/journal.pone.0144653. eCollection 2015. PLoS One. 2015. PMID: 26684752 Free PMC article. - Lysophosphatidylcholine acyltransferase and lysophosphatidylcholine: lysophosphatidylcholine acyltransferase in alveolar type II cells from fetal rat lung.
de Vries AC, Batenburg JJ, van Golde LM. de Vries AC, et al. Biochim Biophys Acta. 1985 Jan 9;833(1):93-9. doi: 10.1016/0005-2760(85)90256-5. Biochim Biophys Acta. 1985. PMID: 4038460 - Phospholipid Remodeling in Physiology and Disease.
Wang B, Tontonoz P. Wang B, et al. Annu Rev Physiol. 2019 Feb 10;81:165-188. doi: 10.1146/annurev-physiol-020518-114444. Epub 2018 Oct 31. Annu Rev Physiol. 2019. PMID: 30379616 Free PMC article. Review. - Biological functions of bacterial lysophospholipids.
Cao X, van Putten JPM, Wösten MMSM. Cao X, et al. Adv Microb Physiol. 2023;82:129-154. doi: 10.1016/bs.ampbs.2022.10.001. Epub 2022 Nov 11. Adv Microb Physiol. 2023. PMID: 36948653 Review.
Cited by
- Lysophospholipid acyltransferases and arachidonate recycling in human neutrophils.
Gijón MA, Riekhof WR, Zarini S, Murphy RC, Voelker DR. Gijón MA, et al. J Biol Chem. 2008 Oct 31;283(44):30235-45. doi: 10.1074/jbc.M806194200. Epub 2008 Sep 3. J Biol Chem. 2008. PMID: 18772128 Free PMC article. - Substrate preferences of a lysophosphatidylcholine acyltransferase highlight its role in phospholipid remodeling.
Kazachkov M, Chen Q, Wang L, Zou J. Kazachkov M, et al. Lipids. 2008 Oct;43(10):895-902. doi: 10.1007/s11745-008-3233-y. Epub 2008 Sep 10. Lipids. 2008. PMID: 18781350 - Biochemistry, physiology, and genetics of GPAT, AGPAT, and lipin enzymes in triglyceride synthesis.
Takeuchi K, Reue K. Takeuchi K, et al. Am J Physiol Endocrinol Metab. 2009 Jun;296(6):E1195-209. doi: 10.1152/ajpendo.90958.2008. Epub 2009 Mar 31. Am J Physiol Endocrinol Metab. 2009. PMID: 19336658 Free PMC article. Review. - DNA Methylome Alterations Are Associated with Airway Macrophage Differentiation and Phenotype during Lung Fibrosis.
McErlean P, Bell CG, Hewitt RJ, Busharat Z, Ogger PP, Ghai P, Albers GJ, Calamita E, Kingston S, Molyneaux PL, Beck S, Lloyd CM, Maher TM, Byrne AJ. McErlean P, et al. Am J Respir Crit Care Med. 2021 Oct 15;204(8):954-966. doi: 10.1164/rccm.202101-0004OC. Am J Respir Crit Care Med. 2021. PMID: 34280322 Free PMC article. - A novel lysophosphatidylcholine acyl transferase activity is expressed by peroxiredoxin 6.
Fisher AB, Dodia C, Sorokina EM, Li H, Zhou S, Raabe T, Feinstein SI. Fisher AB, et al. J Lipid Res. 2016 Apr;57(4):587-96. doi: 10.1194/jlr.M064758. Epub 2016 Feb 1. J Lipid Res. 2016. PMID: 26830860 Free PMC article.
References
- Batenburg J. J. Am. J. Physiol. 1992;262:L367–L385. - PubMed
- Post M., Shuurmans E. A., Batenburg J. J., Van Golde L. M. Biochim. Biophys. Acta. 1983;750:68–77. - PubMed
- Vereyken J. M., Monfoort A., van Golde L. M. Biochim. Biophys. Acta. 1972;260:70–81. - PubMed
- Den Breejen J. N., Batenburg J. J., Van Golde L. M. G. Biochim. Biophys. Acta. 1989;1002:277–282. - PubMed
- Mason R. J., Nellenbogen J. Biochim. Biophys. Acta. 1984;794:392–402. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases