Different mitochondrial intermembrane space proteins are released during apoptosis in a manner that is coordinately initiated but can vary in duration - PubMed (original) (raw)
Different mitochondrial intermembrane space proteins are released during apoptosis in a manner that is coordinately initiated but can vary in duration
Cristina Muñoz-Pinedo et al. Proc Natl Acad Sci U S A. 2006.
Abstract
The release of mitochondrial intermembrane space proteins to the cytosol is a key event during apoptosis. We used in situ fluorescent labeling of proteins tagged with a short tetracysteine-containing sequence to follow the release of Smac, Omi, adenylate kinase-2, cytochrome c, and apoptosis-inducing factor (AIF) during apoptosis and compared the release with that of cytochrome c tagged with GFP in individual cells observed over time. We observed a caspase-independent, simultaneous release of cytochrome c, Smac, Omi, and adenylate kinase-2. Although AIF release also was caspase-independent and commenced with that of the other proteins, it proceeded much more slowly and incompletely from mitochondria, perhaps because of a requirement for a secondary event. These results suggest that these proteins are released through the same mitochondrial pore and that apoptosis may not be regulated through a selective release of individual mitochondrial proteins. The timing and extent of AIF release makes it unlikely that it is involved in the induction of apoptosis, either upstream or downstream of mitochondrial outer membrane permeabilization.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
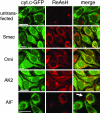
Fig. 1.
TC-tagged proteins display mitochondrial localization. HeLa-cyt.c-GFP cells were stably (Smac, Omi, and AK2) or transiently (AIF) transfected with the vector encoding the corresponding TC-tagged protein and stained with the red dye ReAsH. (Scale bars: 20 μm.) Arrow indicates a cell transfected with AIF-TC; other cells in the field did not express the protein. Full-resolution images are shown in Fig. 5.
Fig. 2.
Smac, AK2, and Omi are released with cytochrome c. (A) HeLa-cyt.c-GFP cells stably expressing Smac-TC were stained with the red dye ReAsH or the green dye FlAsH and treated with the indicated inducers. (A Upper) Scaled average and SD of the punctate/diffuse index are shown of 10 cells from the same experiment, aligned to the time of first detected Smac-FlAsH release (t = 0). Cells also were stained with the mitochondrial dye tetramethylrhodamine ethyl ester (TMRE); TMRE brightness index was calculated by averaging the values of the average intensity of the pixels within each cell. (A Lower) Scaled average of the punctate/diffuse index for Smac-ReAsH and cytochrome c aligned to the time of cytochrome c release (n = 7). Images were taken every 3 min with a ×40 objective. (B) HeLa-cyt.c-GFP cells stably transduced with AK2-TC were stained with ReAsH and treated with the indicated inducers. Graphs show the scaled average of the punctate/diffuse index from 7 (Upper) or 17 (Lower) cells. (C) HeLa-cyt.c-GFP cells were transiently transfected with a construct encoding Omi-TC. Cells were stained with ReAsH, typically 24 to 40 h after transfection, and treated with the indicated inducers. (C Upper) Graph from a representative experiment showing the scaled punctate/diffuse index of cytochrome _c_-GFP and Omi-TC (average and SD of five cells). (C Middle) Graph from an experiment in which cells were treated with staurosporine in the presence of 100 μM zVAD-fmk. Pictures were taken every 3 min. Images from the same experiment are shown in C Lower. Additional cells are shown in Movie 2. (Scale bar: 10 μm.)
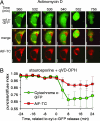
Fig. 3.
AIF-TC is released slowly and in a caspase-independent manner. HeLa-cyt.c-GFP cells were transiently transfected with a construct encoding AIF-TC and stained with the red dye ReAsH typically 24 to 40 h after transfection. Images were taken every 4 min after appropriate incubation times with the inducer, as described in Methods. (A) Time shown is time of total incubation with ActD. (Scale bars: 10 μm.) (B) Average and SD of four cells from an experiment in which cells were treated with staurosporine in the presence of quinoline-Val-Asp-CH2-difluorophenoxy (qVD-OPH).
Fig. 4.
AIF-GFP is released slowly and in a caspase-independent manner. (A_–_C) HeLa cells stably expressing cytochrome _c_-TC were transiently transfected with a construct encoding murine AIF-GFP. Pictures in A show the colocalization of AIF-GFP with the mitochondrial marker Mitotracker Red (MTR). (Scale bars: 10 μm.) (B and C) Cells were stained with the red dye ReAsH to label cytochrome _c_-TC (abbreviated cyt.c-TC) and treated with the indicated inducers. Images were taken every 3 min. Propidium iodide was added to the medium to monitor cell death. (D and E) HeLa cells stably expressing cyt.c-TC were transiently transfected with a construct encoding human AIF-GFP and stained with ReAsH. Graph in D shows the average and SD of punctate/diffuse index for AIF-GFP and cyt.c-TC of six cells aligned to the time of cytochrome c release. (E) Images were taken every 3 min. Numbers show time (in minutes) after drug addition. (Scale bar: 10 μm.)
Similar articles
- Apoptosis is regulated by the VDAC1 N-terminal region and by VDAC oligomerization: release of cytochrome c, AIF and Smac/Diablo.
Shoshan-Barmatz V, Keinan N, Abu-Hamad S, Tyomkin D, Aram L. Shoshan-Barmatz V, et al. Biochim Biophys Acta. 2010 Jun-Jul;1797(6-7):1281-91. doi: 10.1016/j.bbabio.2010.03.003. Epub 2010 Mar 6. Biochim Biophys Acta. 2010. PMID: 20214874 - Toxic proteins released from mitochondria in cell death.
Saelens X, Festjens N, Vande Walle L, van Gurp M, van Loo G, Vandenabeele P. Saelens X, et al. Oncogene. 2004 Apr 12;23(16):2861-74. doi: 10.1038/sj.onc.1207523. Oncogene. 2004. PMID: 15077149 Review. - Endogenously released Smac is insufficient to mediate cell death of human lung carcinoma in response to etoposide.
Bartling B, Lewensohn R, Zhivotovsky B. Bartling B, et al. Exp Cell Res. 2004 Aug 1;298(1):83-95. doi: 10.1016/j.yexcr.2004.04.007. Exp Cell Res. 2004. PMID: 15242764 - Mitochondrial release of pro-apoptotic proteins: electrostatic interactions can hold cytochrome c but not Smac/DIABLO to mitochondrial membranes.
Uren RT, Dewson G, Bonzon C, Lithgow T, Newmeyer DD, Kluck RM. Uren RT, et al. J Biol Chem. 2005 Jan 21;280(3):2266-74. doi: 10.1074/jbc.M411106200. Epub 2004 Nov 9. J Biol Chem. 2005. PMID: 15537572 - Central roles of apoptotic proteins in mitochondrial function.
Kilbride SM, Prehn JH. Kilbride SM, et al. Oncogene. 2013 May 30;32(22):2703-11. doi: 10.1038/onc.2012.348. Epub 2012 Aug 6. Oncogene. 2013. PMID: 22869150 Review.
Cited by
- Rhizoctonia bataticola lectin (RBL) induces caspase-8-mediated apoptosis in human T-cell leukemia cell lines but not in normal CD3 and CD34 positive cells.
Pujari R, Eligar SM, Kumar N, Barkeer S, Reddy V, Swamy BM, Inamdar SR, Shastry P. Pujari R, et al. PLoS One. 2013 Nov 14;8(11):e79311. doi: 10.1371/journal.pone.0079311. eCollection 2013. PLoS One. 2013. PMID: 24244478 Free PMC article. - Glucose metabolism determines resistance of cancer cells to bioenergetic crisis after cytochrome-c release.
Huber HJ, Dussmann H, Kilbride SM, Rehm M, Prehn JH. Huber HJ, et al. Mol Syst Biol. 2011 Mar 1;7:470. doi: 10.1038/msb.2011.2. Mol Syst Biol. 2011. PMID: 21364572 Free PMC article. - New insights into mitochondrial structure during cell death.
Perkins G, Bossy-Wetzel E, Ellisman MH. Perkins G, et al. Exp Neurol. 2009 Aug;218(2):183-92. doi: 10.1016/j.expneurol.2009.05.021. Epub 2009 May 21. Exp Neurol. 2009. PMID: 19464290 Free PMC article. Review. - Molecular mechanisms of cell death: central implication of ATP synthase in mitochondrial permeability transition.
Bonora M, Wieckowski MR, Chinopoulos C, Kepp O, Kroemer G, Galluzzi L, Pinton P. Bonora M, et al. Oncogene. 2015 Mar 19;34(12):1475-86. doi: 10.1038/onc.2014.96. Epub 2014 Apr 14. Oncogene. 2015. PMID: 24727893 Review. - A mitochondria-anchored isoform of the actin-nucleating spire protein regulates mitochondrial division.
Manor U, Bartholomew S, Golani G, Christenson E, Kozlov M, Higgs H, Spudich J, Lippincott-Schwartz J. Manor U, et al. Elife. 2015 Aug 25;4:e08828. doi: 10.7554/eLife.08828. Elife. 2015. PMID: 26305500 Free PMC article.
References
- Danial N. N., Korsmeyer S. J. Cell. 2004;116:205–219. - PubMed
- Green D. R. Cell. 2005;121:671–674. - PubMed
- Verhagen A. M., Ekert P. G., Pakusch M., Silke J., Connolly L. M., Reid G. E., Moritz R. L., Simpson R. J., Vaux D. L. Cell. 2000;102:43–53. - PubMed
- Du C., Fang M., Li Y., Li L., Wang X. Cell. 2000;102:33–42. - PubMed
- Suzuki Y., Imai Y., Nakayama H., Takahashi K., Takio K., Takahashi R. Mol. Cell. 2001;8:613–621. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI52735/AI/NIAID NIH HHS/United States
- AI40646/AI/NIAID NIH HHS/United States
- CA69381/CA/NCI NIH HHS/United States
- P01 CA069381/CA/NCI NIH HHS/United States
- R01 AI040646/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources