Microenvironmental regulators of tissue structure and function also regulate tumor induction and progression: the role of extracellular matrix and its degrading enzymes - PubMed (original) (raw)
Microenvironmental regulators of tissue structure and function also regulate tumor induction and progression: the role of extracellular matrix and its degrading enzymes
M J Bissell et al. Cold Spring Harb Symp Quant Biol. 2005.
Abstract
It is now widely accepted that elements of the cellular and tissue microenvironment are crucial regulators of cell behavior in culture and homeostasis in vivo, and that many of the same factors influence the course of tumor progression. Less well established is the extent to which extracellular factors actually cause cancer, and the circumstances under which this may occur. Using physiologically relevant three-dimensional culture assays and transgenic animals, we have explored how the environmental and architectural context of cells, tissues, and organs controls mammary-specific gene expression, growth regulation, apoptosis, and drug resistance and have found that loss of tissue structure is a prerequisite for cancer progression. Here we summarize this evidence and highlight two of our recent studies. Using mouse mammary epithelial cells, we show that exposure to matrix metalloproteinase-3 (MMP-3) stimulates production of reactive oxygen species (ROS) that destabilize the genome and induce epithelial-mesenchymal transition, causing malignant transformation. Using a human breast cancer progression series, we find that ADAM-dependent growth factor shedding plays a crucial role in acquisition of the malignant phenotype. These findings illustrate how normal tissue structure controls the response to extracellular signals so as to preserve tissue specificity and growth status.
Figures
Figure 1
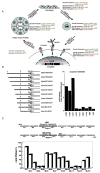
Hierarchical control of tissue function by ECM-dependent cell shape changes, signaling, and morphogenesis. (A) Examples of gene products altered by contact with the ECM and by changes in cellular structure. Studies of MECs cultured in the context of a variety of “designer microenvironments” have demonstrated that cells display distinct behaviors in response to changes in cell shape and ECM composition. In these cultures, the inert substratum, polyHEMA, was used to model cell shape change by itself, whereas purified laminin was used as a ligand that stimulates both cell shape change and integrin-dependent signaling. As cells make the transition from 2D to 3D culture, the expression of distinct cassettes of genes is reciprocally modulated. (B) Deletion analysis of the β-casein promoter identifies an ECM-response element (BCE-1). (C) Systematic mutational analysis of BCE-1 demonstrates that the C/EBPβ and Stat5 binding sites are required for the ECM-dependent transcriptional response. (Modified, with permission, from Schmidhauser et al. 1992; Roskelley et al. 1995; Myers et al. 1998.)
Figure 2
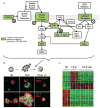
Reversion of the malignant phenotype by modulation of signaling pathways integrated by the ECM. (A) Schematic representation of the signaling pathways downstream of growth factor and adhesion receptors. The activity of some key proteins (green) is required for the maintenance of the malignant phenotype. (B) The malignant phenotype of T4-2 cells may be reverted in 3D ECM culture using inhibitors of the key signaling receptors and their downstream target proteins described above. A representative example (PI3K inhibition) is shown. α6-integrin and ZO-1 are markers of basal and apical polarity, respectively. (C) Hierarchical clustering of Affymetrix microarray data of S1 and T4-2 cells, and T4-2 cells treated with inhibitors of the regulatory proteins shown in A. Reversion of the malignant phenotype using diverse inhibitors elicits a common gene expression pattern (P.A. Kenny and M.J. Bissell, unpubl.) (B, Modified, with permission, from Liu et al. 2004.).
Figure 3
β1-integrin and EGFR protein levels and activities are coordinately modulated in HMT3522 cells in 3D ECM. This reciprocal cross-modulation is not observed in cells cultured on tissue culture plastic. (Reprinted, with permission, from Bissell et al. 1999 [© AACR].)
Figure 4
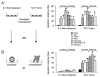
Only nonmalignant cells within mammary acini are resistant to apoptosis. Apoptotic labeling indices calculated for S-1 and T4-2 cells treated with Trail peptide (1 μg/ml), anti-FAS mAb (IgM CH-11, 2 μg/ml), TNF-α (100 nM), etoposide (50 μM), cytochalasin B (1 μM), or paclitaxol (120 nM). Cells were treated (A) as monolayers on a thin coat of collagen I for 24 hr or (B) as 3D structures in rBM for 96 hr. (Reprinted, with permission, from Weaver et al. 2002 [© Elsevier].)
Figure 5
Nonrandom patterns of genomic instability induced by treatment with MMP-3. (A) Analysis of normal and tumor tissue from WAP-MMP-3 transgenic mice by cytogenetic comparative genomic hybridization (CGH) reveals common patterns of deletions (red) in chromosome 4. (B) Cultured SCp2 mouse mammary epithelial cells exposed to MMP-3 become genomically unstable and show deletions in chromosome 4, as revealed by array-based CGH. (A, Adapted from Sternlicht et al. 1999; B, adapted from Radisky et al. 2005.)
Figure 6
Induction of EMT and genomic instability by treatment with MMP-3: Role of Rac1b. (A) MMP-3-treated SCp2 cells, stained for cytokeratins (red), vimentin (green), and DNA (blue); bar, 50 mm. (B) Induction of PALA resistance by MMP-3 (green diamonds, MMP-3; red squares, untreated). (C) Treatment with MMP-3 stimulates production of Rac1b, a highly activated splice isoform of Rac1. (Top) Active Rac species as assessed by PAK pulldown assay; (middle) total cell lysates probed with antibody raised against Rac1b splice insertion; (bottom) total cell lysates probed with Rac1 antibody, showing low levels of Rac1b expression relative to Rac1 in lysate from treated cells. (D) Time course of Rac1b expression in response to MMP-3 treatment and withdrawal. MMP-3 treatment (days 1–4) and washout (days 5–6) (green diamonds, MMP-3-treated; red squares, untreated). (E) Specific suppression of Rac1b by siRNA blocks MMP3-induced EMT. (Adapted from Radisky et al. 2005.)
Figure 7
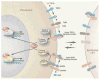
Signaling pathways in stromal cells activated by TGF-β and BMP prevent tumor formation in epithelia. TGF-β signaling can be initiated by association with type III TGF-β receptors prior to formation of an active kinase complex containing receptor types I and II. The active TGF-β signaling complex phosphorylates receptor-regulated R-SMADs such as Smad2 and Smad3, which then bind to common partner co-SMADs such as Smad4. The resulting activated complex can bind to specific DNA sequences and influence the transcription of many tissue-specific genes. Similarly, BMP family members form an active BMP signaling complex, which phosphorylates Smads 1, 5, and 8. These activated R-SMADs then associate with Smad4 to form an active transcriptional complex. Loss of TβRII, Smad4, or BMPRI in stromal cells can stimulate the formation of tumors in adjacent epithelium, although the signals involved in this process have not yet been identified. (Reprinted, with permission, from Radisky and Bissell 2004 [© AAAS].)
Figure 8
Dynamic reciprocity: The whole is greater than the sum of its parts. Gene expression and function of differentiated tissues is an emergent property arising from the bidirectional flow of biochemical and mechanical signals between the ECM and the nucleus, transduced by transmembrane receptors, signaling molecules, and cytoskeletal elements. In the last analysis, however, the organ itself is the unit of function (Bissell and Hall 1987).
Similar articles
- The pathophysiology of epithelial-mesenchymal transition induced by transforming growth factor-beta in normal and malignant mammary epithelial cells.
Taylor MA, Parvani JG, Schiemann WP. Taylor MA, et al. J Mammary Gland Biol Neoplasia. 2010 Jun;15(2):169-90. doi: 10.1007/s10911-010-9181-1. Epub 2010 May 15. J Mammary Gland Biol Neoplasia. 2010. PMID: 20467795 Free PMC article. Review. - In situ force mapping of mammary gland transformation.
Lopez JI, Kang I, You WK, McDonald DM, Weaver VM. Lopez JI, et al. Integr Biol (Camb). 2011 Sep;3(9):910-21. doi: 10.1039/c1ib00043h. Epub 2011 Aug 15. Integr Biol (Camb). 2011. PMID: 21842067 Free PMC article. - The Multi-Faced Role of PAPP-A in Post-Partum Breast Cancer: IGF-Signaling is Only the Beginning.
Jenkins EC, Brown SO, Germain D. Jenkins EC, et al. J Mammary Gland Biol Neoplasia. 2020 Sep;25(3):181-189. doi: 10.1007/s10911-020-09456-1. Epub 2020 Sep 8. J Mammary Gland Biol Neoplasia. 2020. PMID: 32901383 Review. - The role of the microenvironment in mammary gland development and cancer.
Polyak K, Kalluri R. Polyak K, et al. Cold Spring Harb Perspect Biol. 2010 Nov;2(11):a003244. doi: 10.1101/cshperspect.a003244. Epub 2010 Jun 30. Cold Spring Harb Perspect Biol. 2010. PMID: 20591988 Free PMC article. Review. - P-cadherin role in normal breast development and cancer.
Albergaria A, Ribeiro AS, Vieira AF, Sousa B, Nobre AR, Seruca R, Schmitt F, Paredes J. Albergaria A, et al. Int J Dev Biol. 2011;55(7-9):811-22. doi: 10.1387/ijdb.113382aa. Int J Dev Biol. 2011. PMID: 22161837 Review.
Cited by
- Tumor reversion: a dream or a reality.
Tripathi A, Kashyap A, Tripathi G, Yadav J, Bibban R, Aggarwal N, Thakur K, Chhokar A, Jadli M, Sah AK, Verma Y, Zayed H, Husain A, Bharti AC, Kashyap MK. Tripathi A, et al. Biomark Res. 2021 May 6;9(1):31. doi: 10.1186/s40364-021-00280-1. Biomark Res. 2021. PMID: 33958005 Free PMC article. Review. - Stromal epigenetic dysregulation is sufficient to initiate mouse prostate cancer via paracrine Wnt signaling.
Zong Y, Huang J, Sankarasharma D, Morikawa T, Fukayama M, Epstein JI, Chada KK, Witte ON. Zong Y, et al. Proc Natl Acad Sci U S A. 2012 Dec 11;109(50):E3395-404. doi: 10.1073/pnas.1217982109. Epub 2012 Nov 26. Proc Natl Acad Sci U S A. 2012. PMID: 23184966 Free PMC article. - FAM83A confers EGFR-TKI resistance in breast cancer cells and in mice.
Lee SY, Meier R, Furuta S, Lenburg ME, Kenny PA, Xu R, Bissell MJ. Lee SY, et al. J Clin Invest. 2012 Sep;122(9):3211-20. doi: 10.1172/JCI60498. Epub 2012 Aug 13. J Clin Invest. 2012. PMID: 22886303 Free PMC article. - Interconnected contribution of tissue morphogenesis and the nuclear protein NuMA to the DNA damage response.
Vidi PA, Chandramouly G, Gray M, Wang L, Liu E, Kim JJ, Roukos V, Bissell MJ, Moghe PV, Lelièvre SA. Vidi PA, et al. J Cell Sci. 2012 Jan 15;125(Pt 2):350-61. doi: 10.1242/jcs.089177. Epub 2012 Feb 13. J Cell Sci. 2012. PMID: 22331358 Free PMC article. - Activated Abl kinase inhibits oncogenic transforming growth factor-beta signaling and tumorigenesis in mammary tumors.
Allington TM, Galliher-Beckley AJ, Schiemann WP. Allington TM, et al. FASEB J. 2009 Dec;23(12):4231-43. doi: 10.1096/fj.09-138412. Epub 2009 Aug 18. FASEB J. 2009. PMID: 19690215 Free PMC article.
References
- Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, Huang H, Porter D, Hu M, Chin L, Richardson A, Schnitt S, Sellers WR, Polyak K. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6:17. - PubMed
- Ayala F, Corral J, Gonzalez-Conejero R, Sanchez I, Moraleda JM, Vicente V. Genetic polymorphisms of platelet adhesive molecules: Association with breast cancer risk and clinical presentation. Breast Cancer Res Treat. 2003;80:145. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2 R01 CA064786-09/CA/NCI NIH HHS/United States
- R01 CA064786-09/CA/NCI NIH HHS/United States
- R01 CA057621/CA/NCI NIH HHS/United States
- CA57621/CA/NCI NIH HHS/United States
- R01 CA064786/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous