Origin of oligodendrocytes in the subventricular zone of the adult brain - PubMed (original) (raw)
Origin of oligodendrocytes in the subventricular zone of the adult brain
Bénédicte Menn et al. J Neurosci. 2006.
Abstract
Glial fibrillary acidic protein (GFAP)-positive astrocytes (type B cells) in the subventricular zone (SVZ) generate large numbers of new neurons in the adult brain. SVZ stem cells can also generate oligodendrocytes in vitro, but it is not known whether these adult primary progenitors generate oligodendrocytes in vivo. Myelin repair and oligodendrocyte formation in the adult brain is instead associated with glial-restricted progenitors cells, known as oligodendrocyte progenitor cells (OPCs). Here we show that type B cells also generate a small number of nonmyelinating NG2-positive OPCs and mature myelinating oligodendrocytes. Some type B cells and a small subpopulation of actively dividing type C (transit-amplifying) cells expressed oligodendrocyte lineage transcription factor 2 (Olig2), suggesting that oligodendrocyte differentiation in the SVZ begins early in the lineage. Olig2-positive, polysialylated neural cell adhesion molecule-positive, PDGF receptor alpha-positive, and beta-tubulin-negative cells originating in the SVZ migrated into corpus callosum, striatum, and fimbria fornix to differentiate into the NG2-positive nonmyelinating and mature myelinating oligodendrocytes. Furthermore, primary clonal cultures of type B cells gave rise to oligodendrocytes alone or oligodendrocytes and neurons. Importantly, the number of oligodendrocytes derived from type B cells in vivo increased fourfold after a demyelinating lesion in corpus callosum, indicating that SVZ astrocytes participate in myelin repair in the adult brain. Our work identifies SVZ type B cells as progenitors of oligodendrocytes in normal and injured adult brain.
Figures
Figure 1.
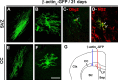
Oligodendrocyte progenitors exist in the SVZ and in the CC of the adult mouse brain. Replication-incompetent murine β-actinp–GFP virus was injected into the SVZ (A–D) or the CC (E, F) of adult CD-1 mice. At 21 d after injection, GFP-labeled mature myelinating (A, E) and nonmyelinating (B–D, F) oligodendrocytes were identified in the CC. C, D, Double immunofluorescence with GFP (green) and Olig2 (C, red) or NG2 (D, red). Note that highly branched GFP+ nonmyelinating oligodendrocytes express both markers. G, Schematic representation of an adult mouse brain hemisphere showing the location of GFP-labeled cells in the CC after β-actinp–GFP injection into the SVZ (red) or the CC (blue). Injection tracks for the SVZ (red) and CC (blue) are represented. Note that GFP-labeled cells are widely dispersed in the adult CC after SVZ retroviral injection in contrast to those generated after injection into the CC. Ctx, Cortex; LV, lateral ventricle; Sep, septum; Str, striatum. Scale bar, 60 μm.
Figure 2.
SVZ astrocytes give rise to oligodendrocytes in the adult mouse brain. RCAS–AP (A–F) or RCAS–GFP (G–L) retroviruses were injected into the SVZ of adult Gtv-a mice, and the progeny of the infected SVZ astrocytes was analyzed 21 d after injection using light (A, B, D, E), confocal (G–L), and electron (C, F) microscopy. A–F, Several AP-labeled cells were identified in the CC (A–D) and in the striatum (E, F): highly branched cells presenting typical morphology of nonmyelinating (A–C) and myelinating (D–F) oligodendrocytes. Characteristics of AP+ nonmyelinating oligodendrocytes (B, C) and AP+ mature myelinating oligodendrocytes (E, F) were determined at the ultrastructural level. B, E, AP-staining in 1.5-μm-thick semithin sections counterstained with toluidine blue. Arrows indicate the nucleus of the cells analyzed at the electron microscopic level. In E, AP+ cell body and processes were traced on top of the picture. C, F, Electron micrograph of AP+ cells. Note the different ultrastructure and typical nuclear heterochromatin in myelinating oligodendrocytes compared with that of the nonmyelinating one. G–L, Double immunofluorescence for GFP (G, J) and the oligodendrocyte markers CNPase (H) or MBP (K) of cells in the CC. Merged field shows combined immunostaining for GFP and CNPase (I) or MBP (L). Arrows indicated SVZ-generated cells in the CC-expressing markers of mature oligodendrocytes. Str, Striatum; V, blood vessel. Scale bar: A, D, 80 μm; B, E, 7 μm; C, F, 2 μm; G–L, 20 μm.
Figure 3.
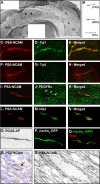
PSA-NCAM-immunopositive cells migrate tangentially within the CC. A, Photomontage of PSA-NCAM immunostaining of whole-mount preparation of the lateral wall of the lateral ventricle and the CC. Dorsal is up, and rostral is left. Filled arrowheads show chains of PSA-NCAM+ cells migrating on the lateral wall of the lateral ventricle. Arrows show PSA-NCAM+ cells migrating within the CC. B, Camera lucida drawing of PSA-NCAM+ cells observed in the CC. These cells are unipolar, with a short leading process, or bipolar. Percentages of cells displaying each morphology are indicated. C–N, Double immunofluorescence for PSA-NCAM (C, F, I, L) and Tuj1 (D, G), PDGFRα (J), or NG2 (M) of cells localized on the lateral wall of the lateral ventricle (C–E) and on the CC (F–N). Merged field shows combined immunostaining for PSA-NCAM and Tuj1 (E, H), PDGFRα (K), or NG2 (N). PSA-NCAM+ cells migrating tangentially as chains expressed the neuronal marker Tuj1 (C–E). In contrast, PSA-NCAM+ cells present at the surface of the CC did not colabel with Tuj1 (F–H) but with the oligodendrocyte markers PDGFRα (I–K) and NG2 (L–N). O–Q, Putative migrating cells located in the CC and labeled after injection of RCAS–AP (O) or β-actinp–GFP (P, Q) into the adult mouse SVZ. Q, Double immunofluorescence for GFP (green) and Olig2 (red) of a bipolar cell. R, S, Electron microscopic characterization of a PSA-NCAM-positive cell in the CC. R, PSA-NCAM staining in toluidine blue-stained 1.5-μm-thick semithin section. Arrow indicates the PSA-NCAM+ cell analyzed at the electron microscopic level. S, Electron micrograph of this PSA-NCAM+ cell. Note that its elongated nucleus contains lax chromatin. Scale bar: A, 400 nm; C–E, 60 μm; F–Q, 50 μm; R, 5 μm; S, 2 μm.
Figure 4.
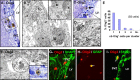
The basic helix–loop–helix transcription factor Olig2 is expressed by a subpopulation of immature transit-amplifying type C cells and by some SVZ astrocytes. Electron microscopic characterization of Olig2+ cells in the adult mouse SVZ. A, C, Inset in F, Olig2 staining in plastic 1.5-μm-thick semithin section counterstained with toluidine blue. Arrows or asterisks indicate Olig2-labeled cells analyzed at the electron microscopic level. B, D, F, Electron micrograph of these cells. Asterisks show the nucleus. A–D, Olig2+ cells display the ultrastructural characteristics of type C cells: medium dark cytoplasm, numerous mitochondria, large Golgi apparatus (arrows in inset in B), and short endoplasmic reticulum (arrowheads in inset in B). Note that some Olig2+ cells have an elongated morphology (C). The cytoplasm of Olig2+ cell is often in contact with myelinated axons (arrows in D). E, Olig2+ cells were grouped in small clusters in the SVZ. Ninety-three Olig2+ cells were analyzed in 1.5-μm-thick sections. The diagram indicates the percentage of Olig2+ cells that were either alone or associated in small groups of two to five cells. nb, Number of. F–I, Olig2+ SVZ cells can also present ultrastructural characteristics of type B SVZ astrocytes that appear to be acquiring some characteristics of type C cells. F, Electron micrograph of such a cell. G–I, This observation was confirmed by performing double immunofluorescence for Olig2 (red) and GFAP (green, G, H) or S100β (green, I) performed on coronal sections of adult mouse brain (G, I) or on freshly dissociated adult SVZ cells (H). Arrows indicate cells expressing both markers. LV, Lateral ventricle. Scale bar: A, C, inset in F, 10 μm; B, D, F, 3 μm; inset in B, 1 μm; G, I, 20 μm; H, 40 μm.
Figure 5.
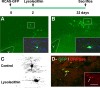
The SVZ astrocytes produce a higher number of oligodendrocytes after a demyelinating lesion. Schematic timeline shows the experimental design. The SVZ astrocytes of GTv-a mice were labeled with RCAS–GFP. Two days later, lysolecithin or vehicle was injected into the ipsilateral CC. The cellular response to demyelination was evaluated 30 d after lesion. A, B, Anti-GFP immunostaining of coronal sections of vehicle-injected (A) and lysolecithin-injected (B) mice. In both cases, mature and immature GFP+ oligodendrocytes were found in the corpus callosum. Insets show GFP+ oligodendrocytes counterstained with the nuclear marker DAPI (4′,6′-diamidino-2-phenylindole) under higher magnification. C, _Z_-stack reconstruction of GFP+ oligodendrocytes found in corpus callosum in control and lysolecithin-lesioned brains. D, Double immunofluorescence for GFP (green) and the CNS myelin protein CNPase (red) of GFP+ cells in the CC of a lesioned brain 30 d after injection of the RCAS–GFP virus. Inset shows the CNPase staining alone in red. Arrows indicate double-labeled cell. V, Lateral ventricle. Scale bar: A, B, 160 μm; insets in A, B, 40 μm; D, inset in D, 25 μm.
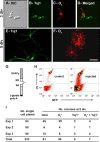
Figure 6.
SVZ astrocytes produce oligodendrocytes in vitro, and some produce both neurons and oligodendrocytes. A–F, Ten thousand SVZ cells isolated from adult CD-1 mice were allowed to differentiate on top of an astrocytic monolayer for 3 div (A–D) and 5 div (E, F). A–D, At 3 div, clusters derived from adult SVZ precursor cells are mixed colonies which contain both O4+ and Tuj1+ cells. E, F, At 5 div, Tuj1+ (E) and O4+ (F) cells present typical morphologies of differentiated neurons and oligodendrocytes, respectively. A, Differential interference contrast (DIC) image of a typical colony. B, E, Immunoreactivity for the neuronal marker Tuj1. C, F, Immunoreactivity for the oligodendroglial marker O4. D, Merged field showing combined immunostaining for Tuj1 and O4. G–I, Single SVZ astrocytes give rise to both neurons and oligodendrocytes in vitro. Adult SVZ astrocytes were specifically labeled in vivo or in vitro with an adenovirus GFAPp–GFP. G, Schematic showing the GFAP promoter reporter construct used for the adenoviral vector. H, I, Single GFP+ SVZ astrocytes were purified by FACS, plated on top of an astrocytic monolayer, and grown in culture for 5 div. H, Density plots showing gates for selecting SVZ astrocytes [GFP and propidium iodide (PI)]. The right plot corresponds to the sorting of SVZ cells isolated at 1.5 d after injection of the adenovirus GFAPp–GFP; the left plot is a control non-injected animal. I, The number of GFP+ cells initially plated is indicated for each experiment (3 independent cultures). The total number of colonies that arose from these cells is presented. These colonies contained only O4+, only Tuj1+, or both O4+ and Tuj1+ cells. Note that 61 of the 233 single GFP+ SVZ cells initially plated gave rise to colonies. Among them, seven generated mixed colonies. Scale bar: A–F, 10 μm. SD, Splice donor; SA, splice acceptor.
Similar articles
- Epidermal growth factor induces the progeny of subventricular zone type B cells to migrate and differentiate into oligodendrocytes.
Gonzalez-Perez O, Romero-Rodriguez R, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Gonzalez-Perez O, et al. Stem Cells. 2009 Aug;27(8):2032-43. doi: 10.1002/stem.119. Stem Cells. 2009. PMID: 19544429 Free PMC article. - Oligodendrogenesis in the subventricular zone and the role of epidermal growth factor.
Gonzalez-Perez O, Alvarez-Buylla A. Gonzalez-Perez O, et al. Brain Res Rev. 2011 Jun 24;67(1-2):147-56. doi: 10.1016/j.brainresrev.2011.01.001. Epub 2011 Jan 12. Brain Res Rev. 2011. PMID: 21236296 Free PMC article. Review. - NG2-expressing cells as oligodendrocyte progenitors in the normal and demyelinated adult central nervous system.
Polito A, Reynolds R. Polito A, et al. J Anat. 2005 Dec;207(6):707-16. doi: 10.1111/j.1469-7580.2005.00454.x. J Anat. 2005. PMID: 16367798 Free PMC article. Review.
Cited by
- Remyelinating Drugs at a Crossroad: How to Improve Clinical Efficacy and Drug Screenings.
Al Jaf AIA, Peria S, Fabiano T, Ragnini-Wilson A. Al Jaf AIA, et al. Cells. 2024 Aug 8;13(16):1326. doi: 10.3390/cells13161326. Cells. 2024. PMID: 39195216 Free PMC article. Review. - Demyelination and impaired oligodendrogenesis in the corpus callosum following lead exposure.
Liu LL, Emir U, Gu H, Sang LT, Sawiak SJ, Cannon JR, Du Y, Zheng W. Liu LL, et al. Toxicol Sci. 2024 Nov 1;202(1):123-141. doi: 10.1093/toxsci/kfae100. Toxicol Sci. 2024. PMID: 39150886 - Single-cell and Spatial Transcriptomics Reveals Ferroptosis as The Most Enriched Programmed Cell Death Process in Hemorrhage Stroke-induced Oligodendrocyte-mediated White Matter Injury.
Gu L, Chen H, Geng R, Sun M, Shi Q, Chen Y, Chang J, Wei J, Ma W, Xiao J, Bao X, Wang R. Gu L, et al. Int J Biol Sci. 2024 Jul 8;20(10):3842-3862. doi: 10.7150/ijbs.96262. eCollection 2024. Int J Biol Sci. 2024. PMID: 39113700 Free PMC article. - Glial cells in the mammalian olfactory bulb.
Zhao D, Hu M, Liu S. Zhao D, et al. Front Cell Neurosci. 2024 Jul 16;18:1426094. doi: 10.3389/fncel.2024.1426094. eCollection 2024. Front Cell Neurosci. 2024. PMID: 39081666 Free PMC article. Review. - Dmd mdx mice have defective oligodendrogenesis, delayed myelin compaction and persistent hypomyelination.
Arreguin AJ, Shao Z, Colognato H. Arreguin AJ, et al. Dis Model Mech. 2024 Apr 1;17(4):dmm050115. doi: 10.1242/dmm.050115. Epub 2024 May 9. Dis Model Mech. 2024. PMID: 38721692 Free PMC article.
References
- Alvarez-Buylla A, Theelen M, Nottebohm F (1990). Proliferation “hot spots” in adult avian ventricular zone reveal radial cell division. Neuron 5:101–109. - PubMed
- Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD (2001). A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci 2:287–293. - PubMed
- Baumann N, Pham-Dinh D (2001). Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev 81:871–927. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous