Factors that can affect the external validity of randomised controlled trials - PubMed (original) (raw)
Factors that can affect the external validity of randomised controlled trials
Peter M Rothwell. PLoS Clin Trials. 2006 May.
No abstract available
Conflict of interest statement
Competing Interests: The author declares that he has no competing interests.
Figures
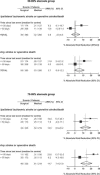
Figure 1. The Absolute Reductions in the Five-Year Risks of Ipsilateral Ischaemic Stroke (Top) and Any Stroke or Death (Bottom) with Surgery in European Carotid Surgery Trial Centres in Which the Median Delay from Last Symptomatic Event to Randomisation Was Less than or Equal to 50 Days (Fast Centres) Compared with Centres with a Longer Delay (Slow Centres)
Data are shown separately for patients with moderate (50%–69%) and severe (70%–99%) carotid stenosis.
Similar articles
- External validity of randomised controlled trials: "to whom do the results of this trial apply?".
Rothwell PM. Rothwell PM. Lancet. 2005 Jan 1-7;365(9453):82-93. doi: 10.1016/S0140-6736(04)17670-8. Lancet. 2005. PMID: 15639683 - Generalisability of clinical trials in otitis media with effusion.
Rovers MM, Zielhuis GA, Bennett K, Haggard M. Rovers MM, et al. Int J Pediatr Otorhinolaryngol. 2001 Jul 30;60(1):29-40. doi: 10.1016/s0165-5876(01)00504-3. Int J Pediatr Otorhinolaryngol. 2001. PMID: 11434951 Clinical Trial. - Is a controlled randomised trial the non-plus-ultra design? A contribution to discussion on comparative, controlled, non-randomised trials.
Gaus W, Muche R. Gaus W, et al. Contemp Clin Trials. 2013 May;35(1):127-32. doi: 10.1016/j.cct.2013.02.011. Epub 2013 Mar 1. Contemp Clin Trials. 2013. PMID: 23459090 - Assessing the validity of clinical trials.
Akobeng AK. Akobeng AK. J Pediatr Gastroenterol Nutr. 2008 Sep;47(3):277-82. doi: 10.1097/MPG.0b013e31816c749f. J Pediatr Gastroenterol Nutr. 2008. PMID: 18728521 Review. - Can findings from randomized controlled trials of social skills training in autism spectrum disorder be generalized? The neglected dimension of external validity.
Jonsson U, Choque Olsson N, Bölte S. Jonsson U, et al. Autism. 2016 Apr;20(3):295-305. doi: 10.1177/1362361315583817. Epub 2015 May 11. Autism. 2016. PMID: 25964654 Review.
Cited by
- How far can we go? A 20-year meta-analysis of dental implant survival rates.
Kupka JR, König J, Al-Nawas B, Sagheb K, Schiegnitz E. Kupka JR, et al. Clin Oral Investig. 2024 Sep 21;28(10):541. doi: 10.1007/s00784-024-05929-3. Clin Oral Investig. 2024. PMID: 39305362 Free PMC article. - Microbiota and probiotics: chances and challenges - a symposium report.
Ruxton CHS, Kajita C, Rocca P, Pot B. Ruxton CHS, et al. Gut Microbiome (Camb). 2023 Mar 27;4:e6. doi: 10.1017/gmb.2023.4. eCollection 2023. Gut Microbiome (Camb). 2023. PMID: 39295904 Free PMC article. Review. - Utilization of geospatial distribution in the measurement of study cohort representativeness.
Feldman K, Kane NJ, Daniels-Young S, Reed B, Welch J, Fitzpatrick L, Hoffman MA, Bradley-Ewing A, Grundberg E. Feldman K, et al. J Biomed Inform. 2024 Sep;157:104687. doi: 10.1016/j.jbi.2024.104687. Epub 2024 Jul 8. J Biomed Inform. 2024. PMID: 38986921 - Vericiguat Use in Patients with Heart Failure in Real-World Settings during the First Year after the Drug Authorization in Japan.
Okami S, Ohlmeier C, Takeichi M, Aguila M, Holl K, Michel A, Lecomte C, Ide T. Okami S, et al. J Clin Med. 2024 May 30;13(11):3222. doi: 10.3390/jcm13113222. J Clin Med. 2024. PMID: 38892932 Free PMC article. - Real-World Evidence to Reinforce Clinical Trial Evidence in Health Technology Assessment: A Critical Review of Real-World Evidence Requirements from Seven Countries and Recommendations to Improve Acceptance.
Thokagevistk K, Coppo C, Rey L, Carelli A, Díez V, Vaselenak S, Oliveira L, Patel A, Sicari E, Ramos T, Schach S, Schirghuber E, Simpson A, Choquet R, Le Lay K. Thokagevistk K, et al. J Mark Access Health Policy. 2024 May 20;12(2):105-117. doi: 10.3390/jmahp12020009. eCollection 2024 Jun. J Mark Access Health Policy. 2024. PMID: 38808313 Free PMC article.
References
- Rothwell PM. External validity of randomised controlled trials: To whom do the results of this trial apply? Lancet. 2005;365:82–93. - PubMed
- Tunis SR, Stryer DB, Clancy CM. Practical clinical trials: Increasing the value of clinical research for decision making in clinical and health policy. JAMA. 2003;290:1624–1632. - PubMed
- European Carotid Surgery Trialists' Collaborative Group. European Carotid Surgery Trialists' Collaborative Group (1998) Randomised trial of endarterectomy for recently symptomatic carotid stenosis: Final results of the MRC European Carotid Surgery Trial (ECST) Lancet. 1998;351:1379–1387. - PubMed
- Fine PEM. Variation in protection by BCG: Implications of and for heterologous immunity. Lancet. 1995;346:1339–1345. - PubMed
- Asymptomatic Carotid Atherosclerosis Study Group. Carotid endarterectomy for patients with asymptomatic internal carotid artery stenosis. JAMA. 1995;273:1421–1428. - PubMed
LinkOut - more resources
Full Text Sources