FK506-binding protein 52 is essential to uterine reproductive physiology controlled by the progesterone receptor A isoform - PubMed (original) (raw)
Comparative Study
. 2006 Nov;20(11):2682-94.
doi: 10.1210/me.2006-0024. Epub 2006 Jul 27.
Irene M Wolf, Hanying Chen, Sumudra Periyasamy, Zhuang Chen, Weidong Yong, Shu Shi, Weihong Zhao, Jianming Xu, Arun Srivastava, Edwin R Sánchez, Weinian Shou
Affiliations
- PMID: 16873445
- PMCID: PMC2661205
- DOI: 10.1210/me.2006-0024
Comparative Study
FK506-binding protein 52 is essential to uterine reproductive physiology controlled by the progesterone receptor A isoform
Zuocheng Yang et al. Mol Endocrinol. 2006 Nov.
Abstract
FK506-binding protein 52 (FKBP52) is a tetratricopeptide repeat protein that associates with steroid receptors in complexes containing heat shock protein 90. To investigate the role of FKBP52 in steroid-regulated physiology, we generated FKBP52-deficient mice. FKBP52 (-/-) females are sterile due to a complete failure of implantation, a process that requires estrogen (ER) and progesterone receptors (PR). Because the uterus expresses two forms of PR, PR-A and PR-B, we investigated all three receptors as potential targets of FKBP52 action. FKBP52 (-/-) uteri showed a normal growth response to estradiol, and unaltered expression of genes controlled by ER and PR-B. In contrast, FKBP52 (-/-) uteri were neither able to express two PR-A-regulated genes, nor undergo decidualization in response to progesterone, suggesting that FKBP52 specifically regulates PR-A at this organ. Analysis of uterine PR heterocomplexes showed preferential association of FKBP52 with PR-A compared with PR-B. Loss of FKBP52 neither disrupted the PR-A/heat shock protein 90 interaction, nor impaired uterine PR-A hormone-binding function, demonstrating the essential role of FKBP52 in PR-A action to be downstream of the hormone-binding event. Transcription studies in +/+ and -/- mouse embryonic fibroblast cells showed a near-complete loss of PR-A activity at mouse mammary tumor virus and synthetic progesterone response element promoters, although partial reductions of ER and PR-B were also observed. Partial disruptions of ovulation and mammary development were also found in FKBP52 (-/-) females. Taken as a whole, our results show FKBP52 to be an essential regulator of PR-A action in the uterus, while being a nonessential but contributory regulator of steroid receptors in the mammary and ovary. These data may now provide the basis for selective targeting of steroid-regulated physiology through tetratricopeptide repeat proteins.
Figures
Fig. 1
Generation of FKBP52-deficient Mice. A, Genomic structure of the mouse FKBP52 gene, targeting vector, and FKBP52 mutant allele. B, Southern blot analysis of FKBP52 genotypes. C, PCR analysis of FKBP52 genotypes. D, Northern blot and E, Western blot analyses confirm the FKBP52 mutant allele to be null. Nonspecific band (NS) serves as a loading control.
Fig. 2
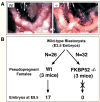
Failure of Implantation in the FKBP52-deficient Female. A, Morphological analysis of uteri from wild-type (Wt) and FKBP52-deficient (−/−) females mated to wild-type males show a complete lack of developing embryos in (−/−) females (E6.5). B, Uterus transfer analysis. Wild-type blastocysts (E3.5) were transferred to wild-type (N=3) and FKBP52-deficient (N=3) pseudo-pregnant females. At E8.5, 17 embryos were recovered from wild-type uteri out of 26 transferred blastocysts (65.4%). In contrast, 0 embryos were recovered from (−/−) uteri out of 32 blastocysts transferred (0%).
Fig. 3
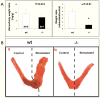
The FKBP52-deficient uterus responds normally to estrogen but fails to undergo decidualization in response to progesterone. A, Comparison of uterus weight following estrogen treatment in ovariectomized FKBP52-deficient and wild-type females. B, Decidualization response. FKBP52-deficient (N=10) and wild-type (N=10) females were ovariectomized and treated with sequential doses of 17β-estradiol and progesterone, as described in Methods. Implantation was mimicked by pricking of one uterine horn with a needle (stimulated). C, Quantitation of results from B.
Fig. 4
FKBP52 deficiency has moderate effects on ovarian and mammary glands functions. A, Partially reduced ovulation in FKBP52 (−/−) females. Young (3–4 weeks of age) FKBP52-deficient (n=8) and wild-type (n=7) females were subjected to a super-ovulation protocol, as described in Methods. B, Mammary gland tertiary ductal side-branching and alveologenesis is partially reduced in FKBP52 (−/−) females. Thoracic whole mounts of mammary glands from untreated (−EP) or 17β-estradiol and progesterone (+EP) treated wild-type (N=6) and FKBP52-deficient (N=10) mice.
Fig. 5
Western Blot Profile of TPR Proteins in Mouse Tissues and Cells. Expression levels of FKBP52, FKBP51, Cyp40 and PP5 were measured in lysates from uterus, ovary, liver and kidney of wild-type mice, as well as wild-type (WT) and FKBP52-deficient (KO) mouse embryonic fibroblast (MEF) cells. Analysis was performed by Western-blotting using lysates of equal protein content.
Fig. 6
Selective Abrogation of PR-A Regulated Genes in Uteri of FKBP52-deficient Females. A, Northern blot analysis of PR-A, PR-B and ER regulated gene expression at pre-implantation (1 day) and post-implantation (3 day) stages in pseudopregnant wild-type and FKBP52-deficient females. Expression of two PR-A regulated genes (calcitonin and amphiregulin) was observed at implantation stage in (+/+) and (+/−) controls, but was absent in FKBP52-deficient uteri. Expression of genes controlled by PR-B (histidine decarboxylase) and ER (lactoferrin) were unaffected in (−/−) uteri. B, Loss of PR-A regulated gene expression in (−/−) uteri is not due to loss of receptor. Western-blot analysis of PR-A and PR-B from wild-type and FKBP52-deficient (KO) uterine lysates. Results from two independent samples are shown.
Fig. 7
Preferential Association of FKBP52 with Uterine PR-A. A, Analysis of FKBP52 content in PR-A and PR-B complexes of the uterus. Uterine lysates from FKBP52 (+/+) and (−/−) females were sequentially immunoadsorbed with antibody against PR-B (PR-6) and antibody against PR-A/B (C-19), followed by Western-blotting with C-19 to detect PR isoforms and HSP56 antibody to detect receptor-bound FKBP52. Nl, non-immune antibody. HC, immunoglobulin heavy chains. Panels for (+/+) and (−/−) co-IPs each represent equal exposures derived from a single autoradiograms. Note that HC bands in the PR-6/C-19 lanes are stronger because counter antibody used is anti-rabbit (to detect C-19), while PR-6 antibody is a mouse monoclonal. B, Quantitation of PR isoforms and receptor-bound FKBP52 from results of Panel A. Densitometric values were normalized as percent of PR-B and represent the mean (+/− SEM) of two experiments. C, Loss of FKBP52 does not alter the PR-A/Hsp90 interaction. PR-A complexes from (+/+) and (−/−) uterine lysates were immunoadsorbed as in Panel A, followed by Western-blotting for PR-A and Hsp90.
Fig. 8
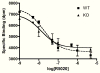
No Alteration of PR-A Hormone-binding Function in FKBP52-deficient Uteri. Uterine lysates from wild-type (WT) and FKBP52-deficient (KO) females were depleted of PR-B by immunoadsorption with PR-6 antibody. Following depletion, a competition hormone-binding assay was performed using 20 nM [3H]R5020 and increasing amounts of unlabeled R5020. Values for specific hormone binding were analyzed by non-linear regression based on one-site competition. EC50 values were not statistically different between WT and KO samples. Analyses of PR-A and PR-B (whole uterine lysates) by saturation hormone binding using [3H]R5020 or [3H]progesterone also showed no differences between genotypes (data not shown).
Fig. 9
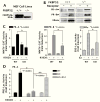
Analysis of PR-A, PR-B and ERα transcription enhancement activities in FKBP52-deficient mouse embryonic fibroblast (MEF) cells. A, Western blot demonstration of presence (+/+) and absence (−/−) of FKBP52 in MEF cell lines. NS, non-specific. B, MEF cells transfected with mPR-A, mPR-B and ERα expression vectors were assayed for receptor expression by Western-blotting. C, Transcriptional enhancement activities in MEF cells of mPR-A and mPR-B at a MMTV-CAT reporter and ERα at an ERE-luciferase reporter. Values represent the means +/− SEM of four (PR-A and PR-B) or six (ERα) independent transfections. D, Activity of PR-A at a PRE-Luc reporter in WT and KO MEF cells and KO MEF cells re-expressing FKBP52 (KO-R52). Values represent the means +/− SEM of four independent transfections.
Similar articles
- Cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation.
Tranguch S, Cheung-Flynn J, Daikoku T, Prapapanich V, Cox MB, Xie H, Wang H, Das SK, Smith DF, Dey SK. Tranguch S, et al. Proc Natl Acad Sci U S A. 2005 Oct 4;102(40):14326-31. doi: 10.1073/pnas.0505775102. Epub 2005 Sep 21. Proc Natl Acad Sci U S A. 2005. PMID: 16176985 Free PMC article. - Proteomic analysis identifies immunophilin FK506 binding protein 4 (FKBP52) as a downstream target of Hoxa10 in the periimplantation mouse uterus.
Daikoku T, Tranguch S, Friedman DB, Das SK, Smith DF, Dey SK. Daikoku T, et al. Mol Endocrinol. 2005 Mar;19(3):683-97. doi: 10.1210/me.2004-0332. Epub 2004 Nov 4. Mol Endocrinol. 2005. PMID: 15528267 - Reproductive functions of progesterone receptors.
Conneely OM, Mulac-Jericevic B, DeMayo F, Lydon JP, O'Malley BW. Conneely OM, et al. Recent Prog Horm Res. 2002;57:339-55. doi: 10.1210/rp.57.1.339. Recent Prog Horm Res. 2002. PMID: 12017551 Review. - Uterine FK506-binding protein 52 (FKBP52)-peroxiredoxin-6 (PRDX6) signaling protects pregnancy from overt oxidative stress.
Hirota Y, Acar N, Tranguch S, Burnum KE, Xie H, Kodama A, Osuga Y, Ustunel I, Friedman DB, Caprioli RM, Daikoku T, Dey SK. Hirota Y, et al. Proc Natl Acad Sci U S A. 2010 Aug 31;107(35):15577-82. doi: 10.1073/pnas.1009324107. Epub 2010 Aug 16. Proc Natl Acad Sci U S A. 2010. PMID: 20713718 Free PMC article. - Role of nuclear progesterone receptor isoforms in uterine pathophysiology.
Patel B, Elguero S, Thakore S, Dahoud W, Bedaiwy M, Mesiano S. Patel B, et al. Hum Reprod Update. 2015 Mar-Apr;21(2):155-73. doi: 10.1093/humupd/dmu056. Epub 2014 Nov 18. Hum Reprod Update. 2015. PMID: 25406186 Free PMC article. Review.
Cited by
- Progesterone Resistance in Endometriosis Is Modulated by the Altered Expression of MicroRNA-29c and FKBP4.
Joshi NR, Miyadahira EH, Afshar Y, Jeong JW, Young SL, Lessey BA, Serafini PC, Fazleabas AT. Joshi NR, et al. J Clin Endocrinol Metab. 2017 Jan 1;102(1):141-149. doi: 10.1210/jc.2016-2076. J Clin Endocrinol Metab. 2017. PMID: 27778641 Free PMC article. - Molecular mechanisms of embryonic implantation in mammals: Lessons from the gene manipulation of mice.
Namiki T, Ito J, Kashiwazaki N. Namiki T, et al. Reprod Med Biol. 2018 Apr 22;17(4):331-342. doi: 10.1002/rmb2.12103. eCollection 2018 Oct. Reprod Med Biol. 2018. PMID: 30377389 Free PMC article. Review. - ARID1A Is Essential for Endometrial Function during Early Pregnancy.
Kim TH, Yoo JY, Wang Z, Lydon JP, Khatri S, Hawkins SM, Leach RE, Fazleabas AT, Young SL, Lessey BA, Ku BJ, Jeong JW. Kim TH, et al. PLoS Genet. 2015 Sep 17;11(9):e1005537. doi: 10.1371/journal.pgen.1005537. eCollection 2015 Sep. PLoS Genet. 2015. PMID: 26378916 Free PMC article. - FKBP4 connects mTORC2 and PI3K to activate the PDK1/Akt-dependent cell proliferation signaling in breast cancer.
Mangé A, Coyaud E, Desmetz C, Laurent E, Béganton B, Coopman P, Raught B, Solassol J. Mangé A, et al. Theranostics. 2019 Sep 21;9(23):7003-7015. doi: 10.7150/thno.35561. eCollection 2019. Theranostics. 2019. PMID: 31660083 Free PMC article. - Proline Isomerization: From the Chemistry and Biology to Therapeutic Opportunities.
Gurung D, Danielson JA, Tasnim A, Zhang JT, Zou Y, Liu JY. Gurung D, et al. Biology (Basel). 2023 Jul 14;12(7):1008. doi: 10.3390/biology12071008. Biology (Basel). 2023. PMID: 37508437 Free PMC article. Review.
References
- Lin RJ, Kao HY, Ordentlich P, Evans RM. The transcriptional basis of steroid physiology. Cold Spring Harb Symp Quant Biol. 1998;63:577–85. - PubMed
- Ing NH, O’Malley BW. The steroid hormone receptor superfamily: molecular mechanisms of action. In: Weintraub BD, editor. Molecular Endocrinology: Basic Concepts and Clinical Correlations. Raven Press, Ltd; New York: 1995. pp. 195–215.
- Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev. 1997;18:306–360. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK73402/DK/NIDDK NIH HHS/United States
- R01 DK073402/DK/NIDDK NIH HHS/United States
- R01 HL070259/HL/NHLBI NIH HHS/United States
- R01 DK070127/DK/NIDDK NIH HHS/United States
- R01 DK073402-02/DK/NIDDK NIH HHS/United States
- R01 DK043867/DK/NIDDK NIH HHS/United States
- DK43867/DK/NIDDK NIH HHS/United States
- HL70259/HL/NHLBI NIH HHS/United States
- DK70127/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous