Tankyrase recruitment to the lateral membrane in polarized epithelial cells: regulation by cell-cell contact and protein poly(ADP-ribosyl)ation - PubMed (original) (raw)
Tankyrase recruitment to the lateral membrane in polarized epithelial cells: regulation by cell-cell contact and protein poly(ADP-ribosyl)ation
Tsung-Yin J Yeh et al. Biochem J. 2006.
Abstract
PARsylation [poly(ADP-ribosyl)ation] of proteins is implicated in the regulation of diverse physiological processes. Tankyrase is a molecular scaffold with this catalytic activity and has been proposed as a regulator of vesicular trafficking on the basis, in part, of its Golgi localization in non-polarized cells. Little is known about tankyrase localization in polarized epithelial cells. Using MDCK (Madin-Darby canine kidney) cells as a model, we found that E-cadherin-mediated intercellular adhesion recruits tankyrase from the cytoplasm to the lateral membrane (including the tight junction), where it stably associates with detergent-insoluble structures. This recruitment is mostly completed within 8 h of calcium-induced formation of cell-cell contact. Conversely, when intercellular adhesion is disrupted by calcium deprivation, tankyrase returns from the lateral membrane to the cytoplasm and becomes more soluble in detergents. The PARsylating activity of tankyrase promotes its dissociation from the lateral membrane as well as its ubiquitination and proteasome-mediated degradation, resulting in an apparent protein half-life of approximately 2 h. Inhibition of tankyrase autoPARsylation using H2O2-induced NAD+ depletion or PJ34 [N-(6-oxo-5,6-dihydrophenanthridin-2-yl)-N,N-dimethylacetamide hydrochloride] treatment results in tankyrase stabilization and accumulation at the lateral membrane. By contrast, stabilization through proteasome inhibition results in tankyrase accumulation in the cytoplasm. These data suggest that cell-cell contact promotes tankyrase association with the lateral membrane, whereas PARsylating activity promotes translocation to the cytosol, which is followed by ubiquitination and proteasome-mediated degradation. Since the lateral membrane is a sorting station that ensures domain-specific delivery of basolateral membrane proteins, the regulated tankyrase recruitment to this site is consistent with a role in polarized protein targeting in epithelial cells.
Figures
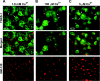
Figure 1. Confocal immunofluorescence analysis of tankyrase (TNKS-1)
Confluent monolayers of MDCK/T1 cells were maintained in the presence (A) or absence (B and C) of doxycycline (Dox) for 2 days to suppress or induce recombinant tankyrase respectively and immunostained for tankyrase (green) and ZO-1 (red). (A) and (B) are confocal images taken at the plane of the TJ. (C) is an XZ image reconstructed from a Z-stack. The co-localization of tankyrase and ZO-1 is shown in yellow in the merged images. Arrowheads, TJs. Arrows, lateral aspect of the basolateral membrane.
Figure 2. Calcium-dependent tankyrase recruitment to the lateral membrane
Recombinant tankyrase was induced for 2 days in confluent MDCK/T1 monolayers maintained in −Dox medium containing CaCl2 at 1.8 mM (A), 100 μM (B) or 5 μM (C). Cells were immunostained for tankyrase (green; upper panels) and GM-130 (red; lower panels) and imaged under a wide-field fluorescence microscope. The merged images (middle panels) show overlapping green and red colours in yellow.
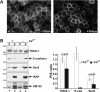
Figure 3. Lateral-membrane localization decreases tankyrase (TNKS-1) solubility in detergents
(A) MDCK/T1−Dox cells were overlaid with PBS (left panel) or PBS containing 1% Triton X-100 (right panel) on ice for 10 min. After removing solubilized proteins, residual proteins were fixed in 4% paraformaldehyde and immunostained for tankyrase. Wide-field images were acquired at the same setting for both samples. (B) Parental MDCK cells were cultured until 1–2 days post-confluence in regular medium, and switched to −Ca or +Ca medium overnight. Monolayers were overlaid with buffer A containing 1% Triton X-100 and 200 mM NaCl [15] (300 μl/2 cm well) at 4 °C for 12 min. Soluble fractions (S) were transferred into a tube containing 100 μl of 4×SDS/PAGE sample buffer. Insoluble residues (P) were detached in 400 μl of 1×SDS sample buffer and homogenized by passing through a 23-gauge needle. Equal volumes (30 μl) of S and P fractions were immunoblotted, and the insoluble/soluble (P/S) ratio was calculated on the basis of densitometric analysis. The bar graph to the right shows the mean P/S ratio (±S.E.M., _n_=4). P values were calculated using Student's two-tailed t test. A representative set of gels is shown to the left. E-cad, E-cadherin.
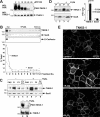
Figure 4. Calcium enhances the membrane association of endogenous tankyrase (TNKS-1) as assayed by iodixanol gradients
Parental MDCK cells grown to confluence in regular medium were switched to −Ca (A and C) or +Ca medium (B) for 38 h. In (C), CaCl2 (1.8 mM) was added back and cells were harvested up to 8 h thereafter. For all samples, postnuclear homogenates (4 mg of protein) were resolved in 30% self-forming iodixanol gradients, and 1 ml fractions were collected from the top for immunoblotting (30 μl/lane). In (A) and (B), the tankyrase and Sec8 contents of each fraction were quantified by densitometry and plotted as a pecentage of the sum of all fractions. In (C), the first fraction [plasma membrane (PM), 60 μl] and the input PNS (30 μg of protein) were immunoblotted and quantified. The PM/PNS ratio at each time point was normalized to the ratio of a monolayer maintained in +Ca medium for 60 h. The results shown are representative of three (A and B) or two (C) independent experiments.
Figure 5. PARP inhibition promotes tankyrase (TNKS-1) association with the lateral membrane
(A) MDCK/T1 monolayers maintained in −Dox medium were treated with PJ34 at the indicated concentrations for 2 h. Whole-cell extracts (10% content of a 2 cm well/lane) were immunoblotted using anti-PAR (upper panel) and anti-tankyrase antibodies (lower panel). WB, Western blot. (B) Parental MDCK cells maintained in +Ca medium in parallel with those used in Figure 4(B) were treated with PJ34 (80 μM) for 2 h. Samples were fractionated in 30% iodixanol gradients and analysed in parallel with Figures 4(A) and 4(B). (C) MDCK/T1 monolayers maintained in −Dox medium were treated with or without H2O2 (1.5 mM; 2 h). PNSs were fractionated in 30% iodixanol gradients as described in (B). The input material (PNS), the top two fractions (F1 and F2), and a mixture of the remaining fractions (F3–F12) were immunoblotted for tankyrase and Sec8 (30 μl each; lanes 1–8). In lanes 9–10, whole-cell extracts were immunoblotted with anti-PAR and anti-tankyrase antibodies. (D) Monolayers of parental MDCK cells were treated with or without PJ34 (80 μM) for 3 h. Triton-soluble (S) and -insoluble (P) pools of tankyrase and Sec8 were determined as in Figure 3(B). The bar graph shows the mean P/S ratio (±S.E.M., _n_=4). A representative set of gels is shown. (E) MDCK/T1 monolayers maintained in −Dox medium were untreated (upper panel) or treated with PJ34 (80 μM, middle panel) or H2O2 (1.5 mM, lower panel) for 2 h. Cells were fixed in methanol/acetone and immunostained for tankyrase. Wide-field immunofluorescence images were acquired at identical settings.
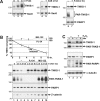
Figure 6. PARP inhibition and proteasome inhibition enhance tankyrase (TNKS-1) stability
(A) Parental MDCK cells (lanes 1–5) and MDCK/T1−Dox cells (lanes 6–7) were treated with PJ34 (80 μM; lanes 2, 3 and 7), 3-AB (5 mM, lane 5) or vehicle only (water for lanes 1 and 6; DMSO for lane 4) for the indicated durations. Cells were lysed in SDS/PAGE sample buffer (400 μl/1 cm well) and samples were immunoblotted (40 μl/lane) for the indicated proteins and for PAR epitopes. (B) Confluent MDCK/T1 monolayers were maintained in −Dox medium for 2 days to induce tankyrase expression. At time zero, cells were treated with doxycycline (100 ng/ml) either alone (lanes 2–4; thick line) or in combination with 80 μM PJ34 (lanes 5–7; dotted line) or 10 μM MG-132 (lanes 8–10; thin line). Whole-cell extracts were harvested at the indicated time points and immunoblotted for tankyrase. The abundance as determined by densitometry (mean±S.E.M., _n_=3) was normalized to time zero and plotted in a semi-logarithmic scale at the top. The same gel was also immunoblotted for PAR epitopes, PARP1 and β-catenin. (C) MDCK/T1−Dox cells grown in −Dox medium were pretreated with H2O2 (1.5 mM; lanes 3–4) or not (lanes 1–2) for 2 h. After rinsing off H2O2 using three washes with −Dox medium, cells were maintained in +Dox (lanes 2 and 4) or −Dox medium (lanes 1 and 3) for 3.5 h. Whole-cell extracts were immunoblotted for PARsylated species and the indicated proteins. Each lane represents 10% of the content from a 1 cm well.
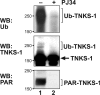
Figure 7. Ubiquitination of tankyrase (TNKS-1)/ is decreased by PJ34
Confluent monolayers of MDCK/T1 were maintained in −Dox medium for 2 days to induce tankyrase expression. After treatment with PJ34 (80 μM; 2 h; lane 2) or without (lane 1), cells were lysed in buffer A (1 ml/15cm plate) supplemented with 1% deoxycholate. Extracts were precleared by incubating with GST protein (50 μg/ml extracts) for 30 min, and centrifuged at 13 000 g for 10 min. Soluble extracts were incubated with GST-IRAP78–108 resins [25] (15 μg of protein/ml extracts) to precipitate tankyrase. The precipitates were immunoblotted using anti-ubiquitin (Ub) (upper panel) and anti-tankyrase (middle panel) antibodies. Extracts were immunoblotted using anti-PAR antibodies (lower panel). WB, Western blot.
Figure 8. Cytoplasmic immunostaining of tankyrase (TNKS-1) is enhanced by MG-132
Monolayers of MDCK/T1 cells maintained in −Dox medium for 2 days to induce tankyrase were untreated (A) or treated with 10 μM MG-132 (B) for 90 min. Cells were co-stained for tankyrase (green) and ZO-1 (red). Wide-field immunofluorescence images were acquired at the same setting for both samples.
Similar articles
- Tankyrase-1 overexpression reduces genotoxin-induced cell death by inhibiting PARP1.
Yeh TY, Sbodio JI, Nguyen MT, Meyer TN, Lee RM, Chi NW. Yeh TY, et al. Mol Cell Biochem. 2005 Aug;276(1-2):183-92. doi: 10.1007/s11010-005-4059-z. Mol Cell Biochem. 2005. PMID: 16132700 - Soluble tankyrase located in cytosol of human embryonic kidney cell line 293.
Kuimov AN, Terekhov SM. Kuimov AN, et al. Biochemistry (Mosc). 2003 Mar;68(3):260-8. doi: 10.1023/a:1023046031434. Biochemistry (Mosc). 2003. PMID: 12733967 - Telomere elongation by a mutant tankyrase 1 without TRF1 poly(ADP-ribosyl)ation.
Muramatsu Y, Tahara H, Ono T, Tsuruo T, Seimiya H. Muramatsu Y, et al. Exp Cell Res. 2008 Mar 10;314(5):1115-24. doi: 10.1016/j.yexcr.2007.12.005. Epub 2007 Dec 14. Exp Cell Res. 2008. PMID: 18221737 - Tankyrases as drug targets.
Lehtiö L, Chi NW, Krauss S. Lehtiö L, et al. FEBS J. 2013 Aug;280(15):3576-93. doi: 10.1111/febs.12320. Epub 2013 Jun 18. FEBS J. 2013. PMID: 23648170 Review. - Poly(ADP-ribosyl)ation reactions in the regulation of nuclear functions.
D'Amours D, Desnoyers S, D'Silva I, Poirier GG. D'Amours D, et al. Biochem J. 1999 Sep 1;342 ( Pt 2)(Pt 2):249-68. Biochem J. 1999. PMID: 10455009 Free PMC article. Review.
Cited by
- First body of evidence suggesting a role of a tankyrase-binding motif (TBM) of vinculin (VCL) in epithelial cells.
Vilchez Larrea S, Valsecchi WM, Fernández Villamil SH, Lafon Hughes LI. Vilchez Larrea S, et al. PeerJ. 2021 May 27;9:e11442. doi: 10.7717/peerj.11442. eCollection 2021. PeerJ. 2021. PMID: 34123588 Free PMC article. - Evidence for tankyrases as antineoplastic targets in lung cancer.
Busch AM, Johnson KC, Stan RV, Sanglikar A, Ahmed Y, Dmitrovsky E, Freemantle SJ. Busch AM, et al. BMC Cancer. 2013 Apr 28;13:211. doi: 10.1186/1471-2407-13-211. BMC Cancer. 2013. PMID: 23621985 Free PMC article. - GDP-mannose-4,6-dehydratase is a cytosolic partner of tankyrase 1 that inhibits its poly(ADP-ribose) polymerase activity.
Bisht KK, Dudognon C, Chang WG, Sokol ES, Ramirez A, Smith S. Bisht KK, et al. Mol Cell Biol. 2012 Aug;32(15):3044-53. doi: 10.1128/MCB.00258-12. Epub 2012 May 29. Mol Cell Biol. 2012. PMID: 22645305 Free PMC article. - PARP-1/2 Inhibitor Olaparib Prevents or Partially Reverts EMT Induced by TGF-β in NMuMG Cells.
Schacke M, Kumar J, Colwell N, Hermanson K, Folle GA, Nechaev S, Dhasarathy A, Lafon-Hughes L. Schacke M, et al. Int J Mol Sci. 2019 Jan 26;20(3):518. doi: 10.3390/ijms20030518. Int J Mol Sci. 2019. PMID: 30691122 Free PMC article. - Hypermetabolism, hyperphagia, and reduced adiposity in tankyrase-deficient mice.
Yeh TY, Beiswenger KK, Li P, Bolin KE, Lee RM, Tsao TS, Murphy AN, Hevener AL, Chi NW. Yeh TY, et al. Diabetes. 2009 Nov;58(11):2476-85. doi: 10.2337/db08-1781. Epub 2009 Aug 3. Diabetes. 2009. PMID: 19651815 Free PMC article.
References
- van der Wouden J. M., Maier O., van IJzendoorn S. C., Hoekstra D. Membrane dynamics and the regulation of epithelial cell polarity. Int. Rev. Cytol. 2003;226:127–164. - PubMed
- Rodriguez-Boulan E., Musch A. Protein sorting in the Golgi complex: shifting paradigms. Biochim. Biophys. Acta. 2005;1744:455–464. - PubMed
- Kreitzer G., Schmoranzer J., Low S. H., Li X., Gan Y., Weimbs T., Simon S. M., Rodriguez-Boulan E. Three-dimensional analysis of post-Golgi carrier exocytosis in epithelial cells. Nat. Cell Biol. 2003;5:126–136. - PubMed
- Rodriguez-Boulan E., Kreitzer G., Musch A. Organization of vesicular trafficking in epithelia. Nat. Rev. Mol. Cell. Biol. 2005;6:233–247. - PubMed
- Polishchuk R., Di Pentima A., Lippincott-Schwartz J. Delivery of raft-associated, GPI-anchored proteins to the apical surface of polarized MDCK cells by a transcytotic pathway. Nat. Cell Biol. 2004;6:297–307. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources