RNA interference screen reveals an essential role of Nedd4-2 in dopamine transporter ubiquitination and endocytosis - PubMed (original) (raw)
RNA interference screen reveals an essential role of Nedd4-2 in dopamine transporter ubiquitination and endocytosis
Tatiana Sorkina et al. J Neurosci. 2006.
Abstract
The function of the dopamine transporter (DAT) to terminate dopamine neurotransmission is regulated by endocytic trafficking of DAT. To elucidate the mechanisms of DAT endocytosis, we generated a fully functional mutant of the human DAT in which a hemagglutinin epitope (HA) was incorporated into the second extracellular loop. The endocytosis assay, based on the uptake of an HA antibody, was designed to study constitutive- and protein kinase C (PKC)-dependent internalization of HA-DAT expressed in non-neuronal cells and rat dopaminergic neurons. Large-scale RNA interference analysis of PKC-dependent endocytosis of HA-DAT revealed the essential and specific role of an E3 ubiquitin ligase, Nedd4-2 (neural precursor cell expressed, developmentally downregulated 4-2), as well as the involvement of adaptor proteins present in clathrin-coated pits, such as epsin, Eps15 (epidermal growth factor pathway substrate clone 15), and Eps15R (Eps15-related protein). Depletion of Nedd4-2 resulted in a dramatic reduction of PKC-dependent ubiquitination of DAT. Endogenous Nedd4-2, epsin, and Eps15 were coimmunoprecipitated with heterologously expressed human HA-DAT and endogenous DAT isolated from rat striatum. A new mechanistic model of DAT endocytosis is proposed whereby the PKC-induced ubiquitination of DAT mediated by Nedd4-2 leads to interaction of DAT with adaptor proteins in coated pits and acceleration of DAT endocytosis.
Figures
Figure 1.
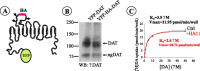
The schematic structure and characterization of YFP-HA-DAT. A, The HA tag was placed in the second extracellular loop of human DAT, which was also tagged with YFP at the N terminus. Predicted _N_-glycosylation sites in the EL2 are indicated. B, Western blot (WB) detection of YFP-DAT and YFP-HA-DAT in lysates of PAE cells. ngDAT, Nonglycosylated YFP-HA-DAT; αDAT, antibody to DAT. C, Kinetic parameters of [3H]DA uptake into PAE/YFP-HA-DAT cells incubated or not incubated (Ctrl) with HA11 were similar to each other and published values for PAE/DAT cells (Sorkina et al., 2003). _K_D, Apparent affinity; Vmax, maximal velocity. The graph is representative of two experiments. Each data point represents an average of three wells.
Figure 2.
Single-cell endocytosis assay using HA11 antibody in PAE cells expressing YFP-HA-DAT. A, Schematics of the endocytosis assay using differential staining of surface and internalized HA11. The cells were incubated with HA11 at 18–22°C for 30 min, washed, further incubated at 37°C, and fixed. The nonpermeabilized cells were then stained with Cy5-tagged anti-mouse secondary IgG to occupy all surface HA11/YFP-HA-DAT complexes. After Triton X-100 (TX-100) permeabilization, the cells were incubated with the same secondary antibody but tagged with Cy3 to mark internalized HA11. B, Representative images of PAE/YFP-HA-DAT in cells treated with vehicle (DMSO) or PMA (1 μ
m
) for 30 min at 37°C after prebinding of HA11. The staining with Cy5 (blue) and Cy3 (red) was performed as described in A. The sum projection images of four consecutive optical sections are presented. Examples in high-magnification insets (single optical sections) demonstrate clusters and compartments containing YFP alone (total YFP-HA-DAT; green arrows), YFP colocalized with Cy5 (plasma membrane HA11; cyan arrows), and YFP-colocalized with Cy3 (internalized HA11; yellow arrows). Identical positions in the insets are indicated by circles. Solid and dashed lines show, respectively, the presence and absence of an endosome or a plasma membrane cluster within the circle. Scale bars, 10 μm. C, Quantification of the ratio of internalized/surface YFP-HA-DAT (mean ± SD; n = 8–9 cells) from the images presented in B (for details, see Results).
Figure 3.
HA11 antibody uptake endocytosis assay in dopaminergic neurons. Mes-Str cultures were transfected with YFP-HA-DAT and assayed after 3 d (see Materials and Methods). The cells were incubated with HA11 for 60 min at 20°C, washed, further incubated for 30 min at 37°C, and fixed. The nonpermeabilized cells were stained with Cy5-tagged anti-mouse secondary IgG. After Triton X-100 permeabilization, the cells were incubated with the same secondary IgG tagged with Cy3 and simultaneously incubated with rabbit antibody to TH. Finally, the cells were incubated with secondary anti-rabbit IgG conjugated with Alexa-350. A _z_-stack of 30 _x_-y images was acquired through four filter channels. The main image (scale bar, 10 μm) and high-magnification (scale bar, 3 μm) images of cell soma (left of the main image) represent optical section 10. An inset at the right bottom corner of the main image represents an image of the TH staining. High-magnification images of a part of the processes (right of the main image) represent optical section 7 (scale bar, 3 μm). Green, cyan, and yellow arrows point to examples of compartments that contain, respectively, only YFP (total DAT), YFP colocalized with Cy5 (surface YFP-HA-DAT), and YFP colocalized with Cy3 (internalized HA11). A white arrow shows an example of an axonal varicosity with overlapping YFP, Cy5, and Cy3 fluorescence.
Figure 4.
RTF siRNA library screening with HeLa/YFP-HA-DAT cells. HeLa/YFP-HA-DAT cells were plated into 96-well plates containing individual or combinations of SMARTpools of siRNA duplexes. After 3 d, the cells were incubated with HA11 for 30 min at 20°C and then for an additional 30 min in the presence of PMA (1 μ
m
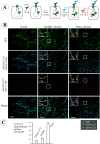
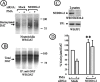
) at 37°C. The cells were fixed and stained with Cy5-labeled secondary (surface), then permeabilized and further stained with Cy-3-labeled secondary (internalized). In a parallel screen, the cells were incubated with 1 μg/ml Tfn-FC for 5 min at 37°C. A, Representative images of YFP-HA-DAT endocytosis in cells transfected with indicated siRNAs. Coordinates of wells are in parentheses. Fluorescence intensity scales are 0–150 for Cy5 and 0–500 for Cy3 for all images. Scale bars, 10 μm. B, Summary of the results based on the visual examination of two independent screens. Essentially identical results were obtained in the two independent screens using two independent cell clones. The efficiency of YFP-HA-DAT and Tfn-FC endocytosis was graded from maximum (+++) (control and mock-transfected cells) to minimum (−; CHC-depleted cells). C, Representative images of Tfn-FC endocytosis in cells transfected with indicated siRNAs. Coordinates of wells are in parentheses. The intensity scale is 0–1000 for all images. Scale bars, 10 μm.
Figure 5.
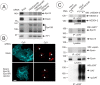
Nedd4–2 is essential for DAT endocytosis and ubiquitination. A, HeLa/YFP-HA-DAT cells were transfected with Nedd4–2 SMARTpool siRNA using conventional forward transfection. The HA11 endocytosis was examined as in Figure 2. Identical intensity settings for fluorescence were used in both images. Scale bars, 10 μm. B, YFP-HA-DAT was immunoprecipitated from mock- and siRNA-transfected HeLa/YFP-HA-DAT cells, and the immunoprecipitates and aliquots of lysates were probed with antibodies to Nedd4–2, ubiquitin, and DAT. DAT-Ub, Ubiquitinated DAT; ng-DAT, nonglycosylated DAT. The lysates were also blotted with antibodies to an unrelated protein (PP1) to control for sample loading. C, PAE/HA-DAT cells were transfected with Nedd4–2-GFP and assayed on the third day. The cells were incubated with HA11 for 60 min at 20°C, washed and fixed, or further incubated with 1 μ
m
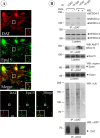
PMA for 30 min at 37°C and then fixed. After fixation, the surface and internalized HA-DAT were stained with Cy5 and Cy3 secondary antibodies as described in Figure 2. The images represent a _z_-sum projection of three optical sections. Arrows show colocalization of HA-DAT and Nedd4–2-GFP in plasma membrane ruffles and cell edges. Insets represent high- magnification images of the cell regions marked by rectangles demonstrating colocalization of Nedd4-GFP and HA11/HA-DAT complexes in endosomes (Cy3 fluorescence). Scale bars, 10 μm.
Figure 6.
Nedd4–2 siRNA reduces PKC-dependent downregulation of untagged DAT. A, HeLa/DAT cells that were transfected twice with control (mock) or Nedd4–2 siRNA and used in experiments 6 d after the first transfection. The cells were incubated with vehicle or PMA as in Figure 2. The cells were then surface biotinylated and lysed, and the biotinylated proteins were pulled-down by NeutrAvidin agarose. Nonbiotinylated DAT was immunoprecipitated with rat anti-DAT from supernatants of NeutrAvidin agarose (B). DAT was detected by Western blotting using rat anti-DAT in NeutrAvidin precipitates (A) and DAT immunoprecipitates (B), whereas Nedd4–2 was probed in aliquots of lysates (C). The lysates were also blotted with antibodies to an unrelated protein (PP1) to control for sample loading (C). Quantification of the amount of biotinylated DAT was performed by densitometry (D). The data represent averaged values from five experiments (±SD) and are expressed as percentage of the amount of biotinylated DAT in vehicle-treated, mock-transfected cells. **p < 0.05 compared with mock-transfected, vehicle-treated cells.
Figure 7.
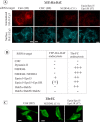
Epsin and Eps15/Eps15R are involved in DAT endocytosis and coimmunoprecipitated with DAT. A, HeLa/YFP-HA-DAT cells were mock transfected or transfected with the mixture of epsin-1 and Eps15 siRNA SMARTpools, or epsin-1 siRNA duplex 2, Eps15 siRNA duplex 3 (Huang et al., 2004), and Eps15R SMARTpool, or with a mixture of SMARTpools of all three siRNAs using conventional forward transfection. Cell lysates were probed with antibodies to Eps15, Esp15R, and epsin-1 to determine the extent of depletion of proteins, and with antibodies to an unrelated protein (PP1) to control for sample loading. B, HA11 endocytosis was examined as in Figure 2. Identical intensity settings for Cy5 or Cy3 fluorescence were used to generate images of mock- versus specific siRNA-transfected cells. The sum projection images of three consecutive optical sections are presented. Scale bars, 10 μm. Asterisks indicate positions of cell nuclei. C, YFP-HA-DAT was immunoprecipitated from parental HeLa or HeLa/YFP-HA-DAT cells (±PMA), and the immunoprecipitates and lysates were probed with antibodies to Nedd4–2, Eps15 and epsin-1, and then with antibodies to DAT.
Figure 8.
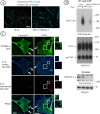
Colocalization of endogenous rat DAT with Eps15 in coated pits and its interaction with Nedd4–2, epsin, and Eps15. A, Mes-Str cultures were fixed and stained with Eps15 and DAT antibodies. High-magnification insets show examples of colocalization of DAT and Eps15 in punctuate structures (coated pits or vesicles) in an axonal widening and varicosities. Scale bars, 5 μm. B, Rat DAT was immunoprecipitated from striatal slices incubated with vehicle (DMSO) or PMA (1 μ
m
) for 30 min at 37°C using goat anti-DAT antibodies. The immunoprecipitates and aliquots of lysates were resolved by electrophoresis and probed with antibodies to Nedd4–2, Eps15, and epsin-1, and then antibodies to DAT. Control normal goat IgG (ns-IgG) was used to control for nonspecific immunoprecipitations.
Similar articles
- Lysine 63-linked polyubiquitination of the dopamine transporter requires WW3 and WW4 domains of Nedd4-2 and UBE2D ubiquitin-conjugating enzymes.
Vina-Vilaseca A, Sorkin A. Vina-Vilaseca A, et al. J Biol Chem. 2010 Mar 5;285(10):7645-56. doi: 10.1074/jbc.M109.058990. Epub 2010 Jan 5. J Biol Chem. 2010. PMID: 20051513 Free PMC article. - Protein kinase C-dependent ubiquitination and clathrin-mediated endocytosis of the cationic amino acid transporter CAT-1.
Vina-Vilaseca A, Bender-Sigel J, Sorkina T, Closs EI, Sorkin A. Vina-Vilaseca A, et al. J Biol Chem. 2011 Mar 11;286(10):8697-8706. doi: 10.1074/jbc.M110.186858. Epub 2011 Jan 5. J Biol Chem. 2011. PMID: 21212261 Free PMC article. - Negative regulation of dopamine transporter endocytosis by membrane-proximal N-terminal residues.
Sorkina T, Richards TL, Rao A, Zahniser NR, Sorkin A. Sorkina T, et al. J Neurosci. 2009 Feb 4;29(5):1361-74. doi: 10.1523/JNEUROSCI.3250-08.2009. J Neurosci. 2009. PMID: 19193883 Free PMC article. - Flotillins regulate membrane mobility of the dopamine transporter but are not required for its protein kinase C dependent endocytosis.
Sorkina T, Caltagarone J, Sorkin A. Sorkina T, et al. Traffic. 2013 Jun;14(6):709-24. doi: 10.1111/tra.12059. Epub 2013 Mar 11. Traffic. 2013. PMID: 23418867 Free PMC article. - Differential targeting of the dopamine transporter to recycling or degradative pathways during amphetamine- or PKC-regulated endocytosis in dopamine neurons.
Hong WC, Amara SG. Hong WC, et al. FASEB J. 2013 Aug;27(8):2995-3007. doi: 10.1096/fj.12-218727. Epub 2013 Apr 23. FASEB J. 2013. PMID: 23612789 Free PMC article.
Cited by
- The role of Nedd4-1 WW domains in binding and regulating human organic anion transporter 1.
Xu D, Wang H, Gardner C, Pan Z, Zhang PL, Zhang J, You G. Xu D, et al. Am J Physiol Renal Physiol. 2016 Aug 1;311(2):F320-9. doi: 10.1152/ajprenal.00153.2016. Epub 2016 May 25. Am J Physiol Renal Physiol. 2016. PMID: 27226107 Free PMC article. - Brain-derived neurotrophic factor (BDNF) enhances GABA transport by modulating the trafficking of GABA transporter-1 (GAT-1) from the plasma membrane of rat cortical astrocytes.
Vaz SH, Jørgensen TN, Cristóvão-Ferreira S, Duflot S, Ribeiro JA, Gether U, Sebastião AM. Vaz SH, et al. J Biol Chem. 2011 Nov 25;286(47):40464-76. doi: 10.1074/jbc.M111.232009. Epub 2011 Oct 3. J Biol Chem. 2011. PMID: 21969376 Free PMC article. - Small molecule induced oligomerization, clustering and clathrin-independent endocytosis of the dopamine transporter.
Sorkina T, Ma S, Larsen MB, Watkins SC, Sorkin A. Sorkina T, et al. Elife. 2018 Apr 9;7:e32293. doi: 10.7554/eLife.32293. Elife. 2018. PMID: 29630493 Free PMC article. - A Physical Interaction between the Dopamine Transporter and DJ-1 Facilitates Increased Dopamine Reuptake.
Luk B, Mohammed M, Liu F, Lee FJ. Luk B, et al. PLoS One. 2015 Aug 25;10(8):e0136641. doi: 10.1371/journal.pone.0136641. eCollection 2015. PLoS One. 2015. PMID: 26305376 Free PMC article. - The _N_-terminal tail of the glycine transporter: role in transporter phosphorylation.
Girotto GL, Vicente CT, Miryam P, Susana B, Alberto PJ, Armando VR, Max S, Manuel M. Girotto GL, et al. Med Res Arch. 2020 May;8(5):2085. doi: 10.18103/mra.v8i5.2085. Epub 2020 May 25. Med Res Arch. 2020. PMID: 37229357 Free PMC article.
References
- Aguilar RC, Watson HA, Wendland B (2003). The yeast Epsin Ent1 is recruited to membranes through multiple independent interactions. J Biol Chem 278:10737–10743. - PubMed
- Benmerah A, Bayrou M, Cerf-Bensussan N, Dautry-Varsat A (1999). Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J Cell Sci 112:1303–1311. - PubMed
- Boehmer C, Wilhelm V, Palmada M, Wallisch S, Henke G, Brinkmeier H, Cohen P, Pieske B, Lang F (2003a). Serum and glucocorticoid inducible kinases in the regulation of the cardiac sodium channel SCN5A. Cardiovasc Res 57:1079–1084. - PubMed
- Boehmer C, Okur F, Setiawan I, Broer S, Lang F (2003b). Properties and regulation of glutamine transporter SN1 by protein kinases SGK and PKB. Biochem Biophys Res Commun 306:156–162. - PubMed
- Boehmer C, Henke G, Schniepp R, Palmada M, Rothstein JD, Broer S, Lang F (2003c). Regulation of the glutamate transporter EAAT1 by the ubiquitin ligase Nedd4–2 and the serum and glucocorticoid-inducible kinase isoforms SGK1/3 and protein kinase B. J Neurochem 86:1181–1188. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous