Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites - PubMed (original) (raw)
Comparative Study
doi: 10.1038/nm1451. Epub 2006 Jul 30.
Affiliations
- PMID: 16892038
- PMCID: PMC1955764
- DOI: 10.1038/nm1451
Comparative Study
Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites
Robert M Anthony et al. Nat Med. 2006 Aug.
Abstract
Although primary and memory responses against bacteria and viruses have been studied extensively, T helper type 2 (T(H)2) effector mechanisms leading to host protection against helminthic parasites remain elusive. Examination of the intestinal epithelial submucosa of mice after primary and secondary infections by a natural gastrointestinal parasite revealed a distinct immune-cell infiltrate after challenge, featuring interleukin-4-expressing memory CD4(+) T cells that induced IL-4 receptor(hi) (IL-4R(hi)) CD206(+) alternatively activated macrophages. In turn, these alternatively activated macrophages (AAMacs) functioned as important effector cells of the protective memory response contributing to parasite elimination, demonstrating a previously unknown mechanism for host protection against intestinal helminths.
Conflict of interest statement
COMPETING INTERESTS STATEMENT
The authors declare that they have no competing financial interests.
Figures
Figure 1
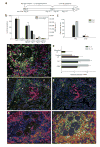
CD4+ T cell–dependent protective mechanisms occur at early stages after Hp challenge inoculation. (a) Anti-CD4 was administered to challenged mice at days 0 and 7(i), 7(ii), 9(iii) or 11(iv) after inoculation. Treatment group number indicated in parentheses. (b) Worm and egg burdens determined 14 d after challenge in mice receiving anti-CD4 treatments. Mean and s.e.m. of five mice per treatment group are shown and are representative of three separate experiments. *P < 0.05 by Kruskal-Wallis test followed by Dunn post hoc test. (c) Parasite larvae recovered from the small intestines of primed or challenged mice using a Baermann apparatus 4 (black bars) and 7 d (gray bars) after infection. Mean and s.e.m. of four or five mice per treatment group are shown and are representative of three separate experiments. * P < 0.001 by Student _t_-test. (d) Fluorescence immunohistochemistry of the host-parasite interface 4 d after challenge, showing IL-4Rαhi (red) cells encircling the parasite, Gr-1+ (green) cells accumulating adjacent to the parasite and CD4+ T (blue) cells surrounding both the parasite and the Gr-1+ population (M, muscularis; Lp, lamina propria; Hp, H. polygyrus). (e) Cytokine mRNA expression levels determined by real-time RT-PCR for CD4+, Gr-1+ and treated lamina propria (CD4− Gr-1−) samples obtained from the host-parasite interface using IF-LCM. Mean and s.e.m. from five mice per treatment group are plotted on a log scale and are representative of two independent experiments. (f) IL-4 protein (green) was detected in situ in the region occupied by CD4+ T cells (blue), which surround Gr-1+ neutrophils (red). (g) Rat IgG1–Alexa488 isotype control antibody showed minimal nonspecific binding in a serial section of the tissue shown in f. (h) F4/80+ macrophages (blue) costained for the IL-4Rα (red), as demonstrated by the ring of purple cells around the parasite. (i) F4/80+ macrophages (red) accumulating at the host-parasite interface expressed CD206 (green), resulting in a yellow-orange stain indicative of their alternatively activated state.
Figure 2
AAMac accumulation 4 d after H. polygyrus challenge is Stat6 dependent. (a,b) Four days after primary infection, Gr-1+ neutrophils (green) accumulated adjacent to the parasite, with substantially fewer CD4+ T cells (blue), IL-4Rhi cells (red), F4/80+ macrophages (b, red) and CD206+ cells (green) compared to what is seen after secondary infection (see Fig. 1d). (c) F4/80+ cells dissected from the host-parasite interface by IF-LCM 4 d after challenge showed gene expression profiles characteristic of AAMacs, including undetectable iNOS (left) and high levels of arginase, Fizz1 (right), Ym1 and AMCase. Mean and s.e.m. of four or five mice per treatment group is plotted on log scales. (d–g) CD4+ T cells transferred from _H. polygyrus_–primed mice induced alternatively activated macrophages in wild-type (d,e) but not _Stat6_-/- recipients (f,g). Gr-1+ neutrophils (red) and CD4+ T cells (blue) accumulated around the parasite 4 d after infection following adoptive transfer of TH2 memory cells into wild-type (d) and _Stat6_-/- (f) recipients. Serial tissue sections show staining for macrophages (F4/80, red) and macrophage mannose receptor (CD206, green), showing that wild-type recipients (e) developed alternatively activated macrophages (yellow), whereas _Stat6_-/- recipients (g) did not. Lp, lamina propria; m, muscularis; Hp, H. polygyrus.
Figure 3
Macrophage depletion abrogates a protective TH2 memory response. (a) Parasite and egg burdens determined 14 d after challenge in mice treated with Cl2MDP or PBS liposomes. (b) Larvae collected using a Baermann apparatus from intestines of challenged mice receiving clodronate or PBS liposome treatment at day 4 and 7 after inoculation. Mean and s.e.m. of five mice per groups are shown; results are representative of three separate experiments. *P < 0.01 by ANOVA followed by Tukey post hoc test. (c–f) Immunofluorescence of the host-parasite interface 4 d after challenge showed that neither clodronate (d) nor PBS liposome (c) treatment affected infiltration of Gr-1+ (red), CD4+ (blue) or CD11c+ (green) cells at the host-parasite interface. F4/80+CD206+ macrophages were specifically depleted in the CL2MDP liposome–treated (f) as compared to the PBS liposome–treated group (e). Cytokine gene expression in mesenteric lymph node (MLN; g, left) and proximal small intestine (right) samples were collected 7 d after infection, as determined by real-time RT-PCR and plotted on log scales. Lp, lamina propria; m, muscularis; Hp, H. polygyrus.
Figure 4
Arginase inhibition abrogates protective memory response to H. polygyrus. (a,b) Shown are parasite and egg burdens determined 14 d after inoculation (a), and larvae recovered from infected small intestines 4 (b, left) or 7 d (right) after infection using a Baermann apparatus, in challenged mice that were given 0.2% BEC (2′HpD14 + BEC) in their drinking water. Bars represent mean and s.e.m. of five mice per group. **P < 0.001 and *P < 0.01 by ANOVA followed by Tukey post hoc test. (c–e) Cytochrome oxidase (black granules) staining of parasite cross-sections 7 d after primary infection (c), secondary infection (d) and secondary infection with BEC treatment (e). Staining intensity within parasite cross-sections was scored blindly (1–4, lightest to darkest). (f) Box plots of scores from four mice per treatment group are plotted. *P < 0.05 by Kruskal-Wallis test followed by Dunn post hoc test.
Similar articles
- Intestinal nematode parasites, cytokines and effector mechanisms.
Else KJ, Finkelman FD. Else KJ, et al. Int J Parasitol. 1998 Aug;28(8):1145-58. doi: 10.1016/s0020-7519(98)00087-3. Int J Parasitol. 1998. PMID: 9762559 Review. - Th2 cytokine-induced alterations in intestinal smooth muscle function depend on alternatively activated macrophages.
Zhao A, Urban JF Jr, Anthony RM, Sun R, Stiltz J, van Rooijen N, Wynn TA, Gause WC, Shea-Donohue T. Zhao A, et al. Gastroenterology. 2008 Jul;135(1):217-225.e1. doi: 10.1053/j.gastro.2008.03.077. Epub 2008 Apr 4. Gastroenterology. 2008. PMID: 18471439 Free PMC article. - Requirements for the development of IL-4-producing T cells during intestinal nematode infections: what it takes to make a Th2 cell in vivo.
Liu Z, Liu Q, Pesce J, Anthony RM, Lamb E, Whitmire J, Hamed H, Morimoto M, Urban JF Jr, Gause WC. Liu Z, et al. Immunol Rev. 2004 Oct;201:57-74. doi: 10.1111/j.0105-2896.2004.00186.x. Immunol Rev. 2004. PMID: 15361233 Review. - Memory Th2 effector cells can develop in the absence of B7-1/B7-2, CD28 interactions, and effector Th cells after priming with an intestinal nematode parasite.
Ekkens MJ, Liu Z, Liu Q, Foster A, Whitmire J, Pesce J, Sharpe AH, Urban JF, Gause WC. Ekkens MJ, et al. J Immunol. 2002 Jun 15;168(12):6344-51. doi: 10.4049/jimmunol.168.12.6344. J Immunol. 2002. PMID: 12055251 - Th2-dependent STAT6-regulated genes in intestinal epithelial cells mediate larval trapping during secondary Heligmosomoides polygyrus bakeri infection.
Westermann S, Schubart C, Dietschmann A, Castiglione K, Radtke D, Voehringer D. Westermann S, et al. PLoS Pathog. 2023 Apr 5;19(4):e1011296. doi: 10.1371/journal.ppat.1011296. eCollection 2023 Apr. PLoS Pathog. 2023. PMID: 37018382 Free PMC article.
Cited by
- Identification of a secreted fatty acid and retinol-binding protein (Hp-FAR-1) from Heligmosomoides polygyrus.
Bath JL, Robinson M, Kennedy MW, Agbasi C, Linz L, Maetzold E, Scheidt M, Knox M, Ram D, Hein J, Clark C, Drees J. Bath JL, et al. J Nematol. 2009 Sep;41(3):228-33. J Nematol. 2009. PMID: 22736819 Free PMC article. - Regulation of the host immune system by helminth parasites.
Maizels RM, McSorley HJ. Maizels RM, et al. J Allergy Clin Immunol. 2016 Sep;138(3):666-675. doi: 10.1016/j.jaci.2016.07.007. Epub 2016 Jul 29. J Allergy Clin Immunol. 2016. PMID: 27476889 Free PMC article. Review. - Adoptive transfer of IL-4Rα+ macrophages is sufficient to enhance eosinophilic inflammation in a mouse model of allergic lung inflammation.
Ford AQ, Dasgupta P, Mikhailenko I, Smith EM, Noben-Trauth N, Keegan AD. Ford AQ, et al. BMC Immunol. 2012 Jan 31;13:6. doi: 10.1186/1471-2172-13-6. BMC Immunol. 2012. PMID: 22292924 Free PMC article. - Worm burden-dependent disruption of the porcine colon microbiota by Trichuris suis infection.
Wu S, Li RW, Li W, Beshah E, Dawson HD, Urban JF Jr. Wu S, et al. PLoS One. 2012;7(4):e35470. doi: 10.1371/journal.pone.0035470. Epub 2012 Apr 20. PLoS One. 2012. PMID: 22532855 Free PMC article. - Neither primary nor memory immunity to Mycobacterium tuberculosis infection is compromised in mice with chronic enteric helminth infection.
Rafi W, Bhatt K, Gause WC, Salgame P. Rafi W, et al. Infect Immun. 2015 Mar;83(3):1217-23. doi: 10.1128/IAI.03004-14. Epub 2015 Jan 20. Infect Immun. 2015. PMID: 25605766 Free PMC article.
References
- Gause WC, Urban JF, Jr, Stadecker MJ. The immune response to parasitic helminths: insights from murine models. Trends Immunol. 2003;24:269–277. - PubMed
- Noel W, Raes G, Hassanzadeh Ghassabeh G, De Baetselier P, Beschin A. Alternatively activated macrophages during parasite infections. Trends Parasitol. 2004;20:126–133. - PubMed
- Mosmann TR, Cherwinski B, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. - PubMed
- Maizels RM, et al. Helminth parasites—masters of regulation. Immunol Rev. 2004;201:89–116. - PubMed
- Zhu Z, et al. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science. 2004;304:1678–1682. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials