Mutational analysis of DJ-1 in Drosophila implicates functional inactivation by oxidative damage and aging - PubMed (original) (raw)
Mutational analysis of DJ-1 in Drosophila implicates functional inactivation by oxidative damage and aging
Marc C Meulener et al. Proc Natl Acad Sci U S A. 2006.
Erratum in
- Proc Natl Acad Sci U S A. 2006 Oct 3;103(40):14978. Thompson, Leonor [corrected to Thomson, Leonor]
Abstract
Inherited mutations in PARK7, the gene encoding DJ-1, are associated with loss of protein function and early-onset parkinsonism. Like human DJ-1 (hDJ-1), Drosophila DJ-1b protects against oxidative insult and is modified with oxidation. We demonstrate that hDJ-1 rescues flies mutant for DJ-1b, and that a conserved cysteine residue in the fly protein (C104, analogous to C106 in hDJ-1) is critical for biological antioxidant function in vivo. Targeted mutagenesis suggests that modification of DJ-1b at this residue inactivates the protective activity of the protein against oxidative stress. Further studies show that DJ-1 modification increases dramatically with age in flies, mice, and humans, with aged flies showing strikingly increased susceptibility to oxidative stress and markedly enhanced DJ-1b modification upon oxidative challenge. Overoxidation of DJ-1 with age and exposure to oxidative toxins may lead to inactivation of DJ-1 function, suggesting a role in susceptibility to sporadic Parkinson's disease.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
Fig. 1.
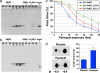
Conservation of the DJ-1 pathway in Drosophila. (a and b) Gel-filtration analysis with samples from flies expressing hDJ-1 (a) or fly DJ-1b (b). Both hDJ-1 and fly DJ-1b eluted at a size consistent with dimer formation: hDJ-1 monomer ≈20 kDa, dimer predicted to be ≈40 kDa; fly DJ-1b monomer ≈19 kDa, dimer ≈38 kDa. (c) Rescue of sensitivity to paraquat of fly DJ-1b deletion mutant (DJ-1bKO, DJ-1b knock-out) with expression of hDJ-1. hDJ-1 was ubiquitously expressed with the driver line da-GAL4 in the background of the DJ-1b deletion (red). Compared to the control (driver alone in DJ-1b deletion background, blue) and to rescue with DJ-1b (DJ-1b expressed with da-GAL4 in the background of DJ-1b deletion, green), hDJ-1 partially rescues paraquat sensitivity. Mean ± SD; ∗, values significantly different from control in blue (P < 0.05, Student’s t test). (d) 2D analysis of hDJ-1 protein in flies exposed to placebo or paraquat-treated food for 10 days. Paraquat exposure increases the level of the more acidic isoform. Bar graph, mean ± SD; ∗, P = 0.015 (Student’s t test).
Fig. 2.
The cysteine residues in DJ-1b are critical for modification by paraquat. (a) Immunoblot (PA636 Ab, 1:1,000) of 2D samples from flies overexpressing wild-type DJ-1b. Three isoforms of DJ-1b are seen; only isoforms 1 and 2 are detectable with endogenous DJ-1b. Isoforms numbered 1, 2, and 3 from basic to acidic. (b) DJ-1b deletion flies expressing wild-type or mutant DJ-1b were exposed to placebo (−) or 2 mM (+) paraquat for 7 days. Samples separated by 2D and analyzed by immunoblot. Isoforms 1 (pI ≈5.8), 2 (pI ≈5.4), and 3 (pI ≈5.2) are indicated. (c) Flies expressing wild-type or mutant DJ-1b in the DJ-1b deletion background were exposed to placebo (−) or 2 mM (+) paraquat for 10 days and samples separated by 1D SDS/PAGE. The 1D shift of DJ-1b with paraquat is not seen with C104A and is constitutive with C104D. (d) Model for the 2D shift of DJ-1b upon oxidative stress. The blue ellipse represents DJ-1b protein, and the two unmodified cysteine residues are denoted in black. Modification of the cysteines is illustrated by a change to red and an asterisk. The data are consistent with isoform 1 representing unmodified DJ-1b; isoform 2, DJ-1b with a single modified cysteine residue of either C45 or C104; and isoform 3, DJ-1b with both cysteine residues modified.
Fig. 3.
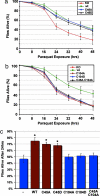
Cysteine 104 is critical for the antioxidant activity of DJ-1b in vivo. DJ-1b deletion mutant flies (KO) expressing DJ-1b mutant proteins with the da-GAL4 driver. The ability of DJ-1b mutant proteins to rescue paraquat sensitivity was assessed with survival curves of flies exposed to 20 mM paraquat. (a) Flies expressing C45A or C45D were fully rescued compared to flies expressing wild-type DJ-1b protein. (b) Flies expressing C104A, C104D, or C45A.C104A are not different from DJ-1b deletion mutant flies. (c) Bar graph showing the percent dead after 24 h, 20 mM paraquat. Mean ± SD; ∗, P < 0.05, compared to DJ-1b deletion (Student’s t test).
Fig. 4.
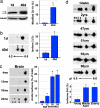
DJ-1 modification increases with age in flies, mice, and humans. (a) Samples from young (1-day-old) and old (40-day-old) flies, run on SDS/PAGE and immunoblotted (PA925 Ab, 1:250). Tubulin, loading control. Black arrow highlights unmodified DJ-1b, and gray arrow indicates modified DJ-1b. Bar graph, percentage of modified DJ-1b, mean ± SD; ∗, P < 0.05 (Student’s t test). (b) Immunoblot of samples from young (1-day-old) and old (40-day-old) flies separated by 2D gel analysis. Bar graph, percentage of DJ-1b present as isoform 2, mean ± SD; ∗, P < 0.05 (Student’s t test). Results were similar in two different isogenic fly lines (BL-5905 and BL-6326). (c) Immunoblot analysis of DJ-1, separated by 2D gel, from forebrain samples of mice of increasing age (4, 12, 18, and 24 months). Results were similar in two different mouse strains (B6D2F1 and C57BL/6). Bar graph, percentage of DJ-1 in acidic isoforms (2, 3, and 4), mean ± SD; ∗, P < 0.05 (Student’s t test). (d) Immunoblot analysis of samples from postmortem human cortex, from individuals of increasing age. Bar graph indicates percentage of hDJ-1 present as isoforms 5 and 6 from each age group (young = 14 weeks to 2 years, middle-aged = 47–51 years, elderly = 92–98 years), mean ± SD, total of nine human brain samples; ∗, P < 0.05 (Student’s t test).
Fig. 5.
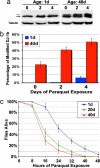
Flies of advanced age display increased DJ-1b modification and increased sensitivity to paraquat. (a) Immunoblot of samples prepared from young (1-day-old) and old (40-day-old) flies exposed to 2 mM paraquat for 0, 2, and 4 days (PA925 Ab, 1:250). Black arrow indicates unmodified DJ-1b, and gray arrow is modified DJ-1b. Tubulin, loading control. (b) Bar graph illustrating the percentage of modified DJ-1b in young (blue) and old (red) flies challenged with paraquat for the indicated number of days. (c) Survival curve of flies of increasing age (1 day old, blue; 20 days old, green; 40 days old, red) exposed to 20 mM paraquat. Results were similar in two isogenic backgrounds [BL-5905 (shown) and BL-6326].
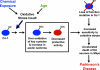
Fig. 6.
Model of DJ-1 loss of function by modification and mutation in Parkinson’s disease. Oxidative stress resulting from environmental exposure or aging leads to oxidation of DJ-1 (blue, active DJ-1) at key cysteine residues (SOx) and the inactivation of DJ-1 biological activity to protect against oxidative stress (red, inactive DJ-1). This is proposed to lead to increased sensitivity to oxidative stress, accelerated loss of dopaminergic (DA) neurons in the substantia nigra pars compacta (SNpc) and contribute to the development of sporadic Parkinson’s disease. In inherited parkinsonism due to loss of DJ-1 gene (top right), cells have increased sensitivity to oxidative stress from initial stages, leading to accelerated loss of dopaminergic neurons and Parkinson’s disease.
Similar articles
- Drosophila DJ-1 mutants are selectively sensitive to environmental toxins associated with Parkinson's disease.
Meulener M, Whitworth AJ, Armstrong-Gold CE, Rizzu P, Heutink P, Wes PD, Pallanck LJ, Bonini NM. Meulener M, et al. Curr Biol. 2005 Sep 6;15(17):1572-7. doi: 10.1016/j.cub.2005.07.064. Curr Biol. 2005. PMID: 16139213 - DJ-1 is critical for mitochondrial function and rescues PINK1 loss of function.
Hao LY, Giasson BI, Bonini NM. Hao LY, et al. Proc Natl Acad Sci U S A. 2010 May 25;107(21):9747-52. doi: 10.1073/pnas.0911175107. Epub 2010 May 10. Proc Natl Acad Sci U S A. 2010. PMID: 20457924 Free PMC article. - Lessons from Drosophila models of DJ-1 deficiency.
Moore DJ, Dawson VL, Dawson TM. Moore DJ, et al. Sci Aging Knowledge Environ. 2006 Jan 11;2006(2):pe2. doi: 10.1126/sageke.2006.2.pe2. Sci Aging Knowledge Environ. 2006. PMID: 16407572 Review. - Effects of pharmacological agents on the lifespan phenotype of Drosophila DJ-1beta mutants.
Lavara-Culebras E, Muñoz-Soriano V, Gómez-Pastor R, Matallana E, Paricio N. Lavara-Culebras E, et al. Gene. 2010 Aug 15;462(1-2):26-33. doi: 10.1016/j.gene.2010.04.009. Epub 2010 Apr 24. Gene. 2010. PMID: 20423725 - DJ-1/PARK7: A New Therapeutic Target for Neurodegenerative Disorders.
Hijioka M, Inden M, Yanagisawa D, Kitamura Y. Hijioka M, et al. Biol Pharm Bull. 2017;40(5):548-552. doi: 10.1248/bpb.b16-01006. Biol Pharm Bull. 2017. PMID: 28458339 Review.
Cited by
- Age- and manganese-dependent modulation of dopaminergic phenotypes in a C. elegans DJ-1 genetic model of Parkinson's disease.
Chen P, DeWitt MR, Bornhorst J, Soares FA, Mukhopadhyay S, Bowman AB, Aschner M. Chen P, et al. Metallomics. 2015 Feb;7(2):289-98. doi: 10.1039/c4mt00292j. Metallomics. 2015. PMID: 25531510 Free PMC article. - Unraveling Parkinson's Disease Neurodegeneration: Does Aging Hold the Clues?
Coleman C, Martin I. Coleman C, et al. J Parkinsons Dis. 2022;12(8):2321-2338. doi: 10.3233/JPD-223363. J Parkinsons Dis. 2022. PMID: 36278358 Free PMC article. Review. - Conservation of oxidative protein stabilization in an insect homologue of parkinsonism-associated protein DJ-1.
Lin J, Prahlad J, Wilson MA. Lin J, et al. Biochemistry. 2012 May 8;51(18):3799-807. doi: 10.1021/bi3003296. Epub 2012 Apr 24. Biochemistry. 2012. PMID: 22515803 Free PMC article. - Altered Ca2+ homeostasis in the skeletal muscle of DJ-1 null mice.
Shtifman A, Zhong N, Lopez JR, Shen J, Xu J. Shtifman A, et al. Neurobiol Aging. 2011 Jan;32(1):125-32. doi: 10.1016/j.neurobiolaging.2009.07.010. Epub 2009 Aug 15. Neurobiol Aging. 2011. PMID: 19683835 Free PMC article. - The role of cysteine oxidation in DJ-1 function and dysfunction.
Wilson MA. Wilson MA. Antioxid Redox Signal. 2011 Jul 1;15(1):111-22. doi: 10.1089/ars.2010.3481. Epub 2011 Jan 14. Antioxid Redox Signal. 2011. PMID: 20812780 Free PMC article. Review.
References
- Lang A. E., Lozano A. M. N. Engl. J. Med. 1998;339:1044–1053. - PubMed
- Dawson T. M., Dawson V. L. Science. 2003;302:819–822. - PubMed
- Paisan-Ruiz C., Jain S., Evans E. W., Gilks W. P., Simon J., van der Brug M., Lopez de Munain A., Aparicio S., Gil A. M., Khan N., et al. Neuron. 2004;44:595–600. - PubMed
- Valente E. M., Abou-Sleiman P. M., Caputo V., Muqit M. M., Harvey K., Gispert S., Ali Z., Del Turco D., Bentivoglio A. R., Healy D. G., et al. Science. 2004;304:1158–1160. - PubMed
- Liou H. H., Tsai M. C., Chen C. J., Jeng J. S., Chang Y. C., Chen S. Y., Chen R. C. Neurology. 1997;48:1583–1588. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous