Duration of the initial TCR stimulus controls the magnitude but not functionality of the CD8+ T cell response - PubMed (original) (raw)
Duration of the initial TCR stimulus controls the magnitude but not functionality of the CD8+ T cell response
Martin Prlic et al. J Exp Med. 2006.
Abstract
CD8+ T cells only require a brief stimulation with antigen in vitro to divide and differentiate into effector and memory cells upon transfer in vivo. The efficiency of clonal expansion and the functional characteristics of memory cells derived from briefly stimulated cells are poorly defined. We developed a system that allowed us to examine programming entirely in vivo. This was achieved by rapidly killing peptide-pulsed DCs carrying a diphtheria toxin receptor transgene with timed injections of diphtheria toxin without altering the course of an accompanying infection. The magnitude of clonal expansion, but not the functionality of the effector cells, correlated directly with the duration of antigen exposure. Furthermore, memory T cells were capable of mounting a secondary response, regardless of the length of antigen encounter during the primary response. These results indicate that the duration of initial antigen encounter influences the magnitude of the primary response, but does not program responsiveness during the secondary challenge.
Figures
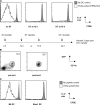
Figure 1.
GFPhi DTR transgenic DCs are rapidly deleted by diphtheria toxin. (A) 3.7 × 105 OVAp-pulsed, GFP-sorted DTRtg DCs were transferred i.v. and the mice were left untreated (left) or treated with DT at 12 h (middle) or 18 h (right) after transfer. 4 × 105 CFSE-labeled OT-I T cells were adoptively transferred 24 h after DC injection, and mice were harvested 3 d after OT-I transfer. Histograms shown are gated on OT-I T cells from an OT-I only control animal (gray) or the respective experimental groups (bold). (B) GFPhi and thus DTRhi-expressing DCs were sorted and used to assure responsiveness to the toxin. Pre-sort FACS plot shown is based on a spleen from a Flt3L-treated animal after depletion of T and B cells (see Material and methods). (C) 8 × 105 DCs from B6 or Kbm1 mice were pulsed with LPS (gray) or LPS + OVAp (bold) and transferred into B6 recipients that received 106 naive OT-I T cells 24 h earlier. 3 d after transfer of the DCs, the CFSE profile of the OT-I cells was used as a readout of T cell activation.
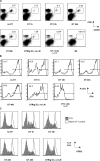
Figure 2.
Varying the antigen exposure time affects the magnitude of the primary CD8+ T cell response. (A) A schematic of the adoptive transfer system. (B) 104 CD45.1 congenic, CFSE-labeled OT-I T cells were transferred with 3.7 × 105 SIINFEKL-pulsed DTRtg DCs, and DT was administered at the indicated time points. All experimental groups apart from the DTRtg DC, OT-I only, and B6 control groups received 2,000 CFU WT-LM. The percentage of OT-I T cells in the spleen is shown. (C) Absolute numbers of OT-I T cells on day 5 of the groups shown in B. Note that the OT-I only animals received 100× more OT-I T cells than animals in the experimental groups.
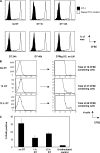
Figure 3.
Varying the antigen exposure time does not affect CD8+ T cell functionality. (A) To determine trafficking properties, lungs of mice were harvested after perfusion of the animal and analyzed for the presence of OT-I T cells. The percentage of OT-I T cells in the lung is shown. (B) Splenocytes were tested for their ability to produce IFNγ in a 4-h assay. Histograms are based on a gate specific for the congenic OT-I T cells. (C) Splenic OT-I T cells were analyzed for CD62L surface expression on day 5. Naive OT-I control cells are shown in white, and OT-I T cells from the experimental groups are shown in gray.
Figure 4.
Proliferation but not recruitment contributes to the different size of the OT-I pool in DT-treated animals. (A) The CFSE profile of OT-I T cells in all groups was analyzed on day 5 after priming (black histogram) and compared with an unprimed control (white histogram). (B) To further assess whether unrecruited, CFSE high cells are present in our early DT-treated groups, a pull-down assay was performed (see Materials and methods). The panels on the left are gated on OT-I T cells from untreated (top), 1 h–treated (middle), or 12 h–treated (bottom) mice. On the right, the same data are shown on a bigger scale to facilitate detection of low numbers of cells. (C) Mice were pulsed with BrdU 2 h before harvesting on day 5. Congenic OT-I T cells were analyzed for their BrdU content.
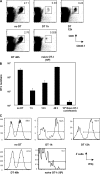
Figure 5.
Shortening antigen exposure time in the primary response does not affect the efficiency of the secondary response. (A) 35 d after priming, mice were rechallenged with a dose of 2 × 105 LM-OVA, harvested 3 d after rechallenge, and the magnitude of the recall response was assessed by analyzing the size of the OT-I pool. The naive OT-I group that had received 106 OT-I on day 0 remained uninfected. The percentage of OT-I T cells in the spleen is shown. (B) Based on the data shown in A, the absolute number of OT-I T cells was calculated. (C) Splenocytes were tested for their ability to produce IFNγ in a 4-h in vitro assay. Histograms are based on a gate specific for the congenic OT-I T cells.
Similar articles
- Differential kinetics of antigen dependency of CD4+ and CD8+ T cells.
Rabenstein H, Behrendt AC, Ellwart JW, Naumann R, Horsch M, Beckers J, Obst R. Rabenstein H, et al. J Immunol. 2014 Apr 15;192(8):3507-17. doi: 10.4049/jimmunol.1302725. Epub 2014 Mar 17. J Immunol. 2014. PMID: 24639353 - Stimulation of CD8+ T cells following diphtheria toxin-mediated antigen delivery into dendritic cells.
Shaw CA, Starnbach MN. Shaw CA, et al. Infect Immun. 2006 Feb;74(2):1001-8. doi: 10.1128/IAI.74.2.1001-1008.2006. Infect Immun. 2006. PMID: 16428746 Free PMC article. - Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naïve cells.
Kaech SM, Ahmed R. Kaech SM, et al. Nat Immunol. 2001 May;2(5):415-22. doi: 10.1038/87720. Nat Immunol. 2001. PMID: 11323695 Free PMC article.
Cited by
- The role of naive T cell precursor frequency and recruitment in dictating immune response magnitude.
Jenkins MK, Moon JJ. Jenkins MK, et al. J Immunol. 2012 May 1;188(9):4135-40. doi: 10.4049/jimmunol.1102661. J Immunol. 2012. PMID: 22517866 Free PMC article. Review. - Enhancing mucosal immunity by transient microbiota depletion.
Becattini S, Littmann ER, Seok R, Amoretti L, Fontana E, Wright R, Gjonbalaj M, Leiner IM, Plitas G, Hohl TM, Pamer EG. Becattini S, et al. Nat Commun. 2020 Sep 8;11(1):4475. doi: 10.1038/s41467-020-18248-4. Nat Commun. 2020. PMID: 32901029 Free PMC article. - Sustained antigen presentation can promote an immunogenic T cell response, like dendritic cell activation.
Obst R, van Santen HM, Melamed R, Kamphorst AO, Benoist C, Mathis D. Obst R, et al. Proc Natl Acad Sci U S A. 2007 Sep 25;104(39):15460-5. doi: 10.1073/pnas.0707331104. Epub 2007 Sep 19. Proc Natl Acad Sci U S A. 2007. PMID: 17881563 Free PMC article. - Duration of antigen expression in vivo following DNA immunization modifies the magnitude, contraction, and secondary responses of CD8+ T lymphocytes.
Hovav AH, Panas MW, Rahman S, Sircar P, Gillard G, Cayabyab MJ, Letvin NL. Hovav AH, et al. J Immunol. 2007 Nov 15;179(10):6725-33. doi: 10.4049/jimmunol.179.10.6725. J Immunol. 2007. PMID: 17982062 Free PMC article. - Regulation of T Helper Cell Fate by TCR Signal Strength.
Bhattacharyya ND, Feng CG. Bhattacharyya ND, et al. Front Immunol. 2020 May 19;11:624. doi: 10.3389/fimmu.2020.00624. eCollection 2020. Front Immunol. 2020. PMID: 32508803 Free PMC article. Review.
References
- Gourley, T.S., E.J. Wherry, D. Masopust, and R. Ahmed. 2004. Generation and maintenance of immunological memory. Semin. Immunol. 16:323–333. - PubMed
- Curtsinger, J.M., C.S. Schmidt, A. Mondino, D.C. Lins, R.M. Kedl, M.K. Jenkins, and M.F. Mescher. 1999. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J. Immunol. 162:3256–3262. - PubMed
- Curtsinger, J.M., J.O. Valenzuela, P. Agarwal, D. Lins, and M.F. Mescher. 2005. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J. Immunol. 174:4465–4469. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials