Inhibition of phosphatidylinositol 3-kinase destabilizes Mycn protein and blocks malignant progression in neuroblastoma - PubMed (original) (raw)
Inhibition of phosphatidylinositol 3-kinase destabilizes Mycn protein and blocks malignant progression in neuroblastoma
Louis Chesler et al. Cancer Res. 2006.
Erratum in
- Cancer Res. 2006 Oct 15;66(20):10227
- Editor's Note: Inhibition of Phosphatidylinositol 3-Kinase Destabilizes Mycn Protein and Blocks Malignant Progression in Neuroblastoma.
Chesler L, Schlieve C, Goldenberg DD, Kenney A, Kim G, McMillan A, Matthay KK, Rowitch D, Weiss WA. Chesler L, et al. Cancer Res. 2024 Oct 1;84(19):3311. doi: 10.1158/0008-5472.CAN-24-2888. Cancer Res. 2024. PMID: 39350668 No abstract available.
Abstract
Amplification of MYCN occurs commonly in neuroblastoma. We report that phosphatidylinositol 3-kinase (PI3K) inhibition in murine neuroblastoma (driven by a tyrosine hydroxylase-MYCN transgene) led to decreased tumor mass and decreased levels of Mycn protein without affecting levels of MYCN mRNA. Consistent with these observations, PI3K inhibition in MYCN-amplified human neuroblastoma cell lines resulted in decreased levels of Mycn protein without affecting levels of MYCN mRNA and caused decreased proliferation and increased apoptosis. To clarify the importance of Mycn as a target of broad-spectrum PI3K inhibitors, we transduced wild-type N-myc and N-myc mutants lacking glycogen synthase kinase 3beta phosphorylation sites into human neuroblastoma cells with no endogenous expression of myc. In contrast to wild-type N-myc, the phosphorylation-defective mutant proteins were stabilized and were resistant to the antiproliferative effects of PI3K inhibition. Our results show the importance of Mycn as a therapeutic target in established tumors in vivo, offer a mechanistic rationale to test PI3K inhibitors in MYCN-amplified neuroblastoma, and represent a therapeutic approach applicable to a broad range of cancers in which transcription factors are stabilized through a PI3K-dependent mechanism.
Figures
Figure 1
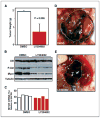
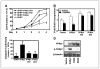
Inhibition of PI3K blocks growth of neuroblastoma in vivo. Tumor-bearing animals transgenic for TH-MYCN were treated at ~60 days of life using 50 mg/kg LY294002 (n = 7) or vehicle (n = 6). Treatment was by daily i.p. injection for 12 days, at which time animals were sacrificed. A, average weight of resected tumors. B, Western analysis of five separate tumors shows decreased levels of Mycn and pAkt in tumor lysates from treated animals. C, quantitative reverse transcription-PCR (RT-PCR; Taqman) analysis of MYCN mRNA levels from four tumors treated with LY294002 or vehicle shows no significant differences in levels of MYCN mRNA (43.4% versus 38.7% of GAPDH expression; χ2 = 0.138; P ≤ 1). D, tumor from a representative animal treated with DMSO, showing a large abdominal primary tumor (T) encapsulating one kidney (K). E, tumor from a representative animals treated with LY294002, showing shrinkage of tumor, with a more discrete primary tumor mass adjacent to the kidney.
Figure 2
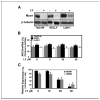
LY294002 treatment reduces steady-state levels of Mycn protein in human neuroblastoma cells. A, to assess the effect of LY294002 (LY) on steady-state levels of Mycn, neuroblastoma cell lines were grown in 10% FBS and treated with LY294002 (20 μmol/L) for 24 hours. Lysates were immunoblotted with antisera against Mycn protein or β-tubulin. Tet21/N cells are a derivative of SHEP neuroblastoma cells that stably express MYCN under control of the tetracycline system. Kelly and LAN-1 are tumor-derived cell lines amplified for MYCN. B, quantitative RT-PCR (Taqman) analysis of MYCN mRNA levels from Tet21/N, Kelly, and LAN-1 cells treated in (A) showed no significant effect of LY294002 on MYCN mRNA expression. Average of three separate conditions. C, LY294002-induced destabilization of Mycn was associated with inhibition of the known downstream Mycn targets MDM2 (23) and MCM7 (24). Levels of these targets were assessed by Taqman analysis under the same experimental conditions as in (A).
Figure 3
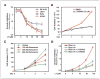
PI3K inhibition blocks accumulation of viable neuroblastoma cells. A, dose response of human neuroblastoma cell lines to PI3K inhibition. Neuroblastoma cell lines were treated with LY294002 at dosages shown and analyzed at 24 hours by water-soluble tetrazolium (WST-1) assay. SK-N-SH cells are diploid for MYCN. The other cell lines show amplification of MYCN. B and C, PI3K inhibition leads to decreased viability. The human neuroblastoma cell line Kelly was grown in the presence of LY294002 (B), wortmannin (C), or DMSO vehicle control. Wortmannin was added to medium twice daily. Viable trypan blue–excluding cells were counted on days shown. D, efficacy of PI3K-mediated proliferation block is dependent on Mycn. Tet21/N cells were treated with LY294002 or vehicle in the absence or presence of doxycycline. Decreased accumulation of viable cells mediated by LY294002 (hemacytometer counting) was most pronounced in cells that expressed MYCN. A to D, average of three separate experiments.
Figure 4
Inhibition of PI3K induces apoptosis of neuroblastoma cells. A, LAN-1 cells were treated with vehicle or LY294002 (10 μmol/L) for 24 hours. TUNEL-stained cells were visualized by confocal microscopy and quantitated (standardized to camptothecin = 100% apoptosis). B, as an independent measure of apoptosis, adherent LAN-1 neuroblastoma cells were examined by cell death detection ELISA at 24 hours as a function of LY294002 dosage. C, to assess toxicity of LY294002 treatment, Kelly cells were treated with LY294002 at doses indicated. DNA release into the culture medium was assessed at 24 hours using a dual-antibody ELISA to histone H2b to assay for cellular necrosis. Toxicity of LY294002 was detected above a dose of 25 μmol/L. Average of three separate experiments.
Figure 5
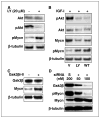
Inhibition of PI3K causes increased phosphorylation and decreased levels of Mycn protein. A, Kelly cells were treated with 20 μmol/L LY294002 for 24 hours in 10% serum, with lactacystin (10 μmol/L) added at 3 hours. Immunoblots show increased levels of pMycn and decreased levels of pAkt. B, serum-starved SH-SY5Y cells were treated for 6 hours with vehicle (V; DMSO), LY294002, or wortmannin in the presence of IGF-I and lactacystin. IGF-1 led to increased levels of pAkt. LY294002 and wortmannin blocked pAkt, resulting in increased levels of pMycn and decreased levels of total Mycn. C, chemical inhibition of GSK3β (SH-SY5Y cells, GSK inhibitor II) led to an increase in the level of total Mycn protein. D, siRNA-mediated inhibition of GSK3β leads to reduced levels of pMycn and increased levels of total Mycn, consistent with GSK3β as the kinase that directly phosphorylates and destabilizes Mycn. Kelly cells growing in serum were transiently transfected with siRNA directed against GSK3β or a scrambled siRNA control (S). Levels of GSK3β, pGSK3β, Mycn, and pMycn were assessed at 24 hours.
Figure 6
Nonphosphorylable mutants of N-myc are stabilized and resistant to PI3K inhibition. SHEP cells were stably transduced with wild-type N-myc or N-myc constructs mutant at the NH2-terminal GSK3β phosphorylation sites. A to C, SHEP cells stably transduced with phosphorylation-defective mutants of N-myc grew more efficiently in the presence of serum (hemacytometer counting assay; A), incorporated more BrdUrd in the presence and absence of LY294002 (B), and were resistant to the proapoptotic effects of LY294002 (caspase-3 cleavage assay; C) in comparison with cells transduced with wild-type N-myc. D, Western analysis shows higher levels of N-myc:T50A and N-myc:S54A in contrast to wild-type N-myc protein. The two mutant N-myc proteins were also not phosphorylated appreciably (using antisera that recognizes both phosphorylation sites in N-myc; ref. 30).
Figure 7
PI3K blockade reduces the half-life of Mycn protein. A, treatment of Kelly cells with cycloheximide verifies that LY294002 acts post-transcriptionally to destabilize Mycn protein. Cells were treated with 20 μmol/L LY294002 or vehicle for 24 hours before the addition of cycloheximide. LY294002 treatment led to rapid degradation of Mycn (_t_1/2, 1.2 hours) compared with untreated controls (_t_1/2, 2.3 hours). B, treatment of SHEP cells with cycloheximide verifies that phosphorylation-defective N-myc proteins show increased stability in the presence of LY294002. Cells were treated with as in (A) and harvested at the times shown (after cycloheximide addition). LY294002 treatment led to rapid degradation of N-myc to levels comparable with that in (A) (_t_1/2, 1.2 hours). Phosphorylation-defective mutants of N-myc were stabilized dramatically, with half-lives 6.5 hours for the T50 mutant and >9 hours for the S54 mutant proteins. See Materials and Methods for details.
Similar articles
- The aurora kinase inhibitor CCT137690 downregulates MYCN and sensitizes MYCN-amplified neuroblastoma in vivo.
Faisal A, Vaughan L, Bavetsias V, Sun C, Atrash B, Avery S, Jamin Y, Robinson SP, Workman P, Blagg J, Raynaud FI, Eccles SA, Chesler L, Linardopoulos S. Faisal A, et al. Mol Cancer Ther. 2011 Nov;10(11):2115-23. doi: 10.1158/1535-7163.MCT-11-0333. Epub 2011 Sep 1. Mol Cancer Ther. 2011. PMID: 21885865 Free PMC article. - Molecular rationale for the use of PI3K/AKT/mTOR pathway inhibitors in combination with crizotinib in ALK-mutated neuroblastoma.
Moore NF, Azarova AM, Bhatnagar N, Ross KN, Drake LE, Frumm S, Liu QS, Christie AL, Sanda T, Chesler L, Kung AL, Gray NS, Stegmaier K, George RE. Moore NF, et al. Oncotarget. 2014 Sep 30;5(18):8737-49. doi: 10.18632/oncotarget.2372. Oncotarget. 2014. PMID: 25228590 Free PMC article. - The MYCN oncoprotein as a drug development target.
Lu X, Pearson A, Lunec J. Lu X, et al. Cancer Lett. 2003 Jul 18;197(1-2):125-30. doi: 10.1016/s0304-3835(03)00096-x. Cancer Lett. 2003. PMID: 12880971 Review. - Hsp90 inhibition increases p53 expression and destabilizes MYCN and MYC in neuroblastoma.
Regan PL, Jacobs J, Wang G, Torres J, Edo R, Friedmann J, Tang XX. Regan PL, et al. Int J Oncol. 2011 Jan;38(1):105-12. Int J Oncol. 2011. PMID: 21109931 Free PMC article. - MDM2 as MYCN transcriptional target: implications for neuroblastoma pathogenesis.
Slack A, Lozano G, Shohet JM. Slack A, et al. Cancer Lett. 2005 Oct 18;228(1-2):21-7. doi: 10.1016/j.canlet.2005.01.050. Cancer Lett. 2005. PMID: 15927364 Review.
Cited by
- Aggressive neuroblastomas have high p110alpha but low p110delta and p55alpha/p50alpha protein levels compared to low stage neuroblastomas.
Fransson S, Kogner P, Martinsson T, Ejeskär K. Fransson S, et al. J Mol Signal. 2013 Apr 18;8(1):4. doi: 10.1186/1750-2187-8-4. J Mol Signal. 2013. PMID: 23597230 Free PMC article. - The aurora kinase inhibitor CCT137690 downregulates MYCN and sensitizes MYCN-amplified neuroblastoma in vivo.
Faisal A, Vaughan L, Bavetsias V, Sun C, Atrash B, Avery S, Jamin Y, Robinson SP, Workman P, Blagg J, Raynaud FI, Eccles SA, Chesler L, Linardopoulos S. Faisal A, et al. Mol Cancer Ther. 2011 Nov;10(11):2115-23. doi: 10.1158/1535-7163.MCT-11-0333. Epub 2011 Sep 1. Mol Cancer Ther. 2011. PMID: 21885865 Free PMC article. - New strategies in neuroblastoma: Therapeutic targeting of MYCN and ALK.
Barone G, Anderson J, Pearson AD, Petrie K, Chesler L. Barone G, et al. Clin Cancer Res. 2013 Nov 1;19(21):5814-21. doi: 10.1158/1078-0432.CCR-13-0680. Epub 2013 Aug 21. Clin Cancer Res. 2013. PMID: 23965898 Free PMC article. Review. - Anti-tumor effects of PIM/PI3K/mTOR triple kinase inhibitor IBL-302 in neuroblastoma.
Mohlin S, Hansson K, Radke K, Martinez S, Blanco-Apiricio C, Garcia-Ruiz C, Welinder C, Esfandyari J, O'Neill M, Pastor J, von Stedingk K, Bexell D. Mohlin S, et al. EMBO Mol Med. 2019 Aug;11(8):e10058. doi: 10.15252/emmm.201810058. Epub 2019 Jul 16. EMBO Mol Med. 2019. PMID: 31310053 Free PMC article. - EGFR signals to mTOR through PKC and independently of Akt in glioma.
Fan QW, Cheng C, Knight ZA, Haas-Kogan D, Stokoe D, James CD, McCormick F, Shokat KM, Weiss WA. Fan QW, et al. Sci Signal. 2009 Jan 27;2(55):ra4. doi: 10.1126/scisignal.2000014. Sci Signal. 2009. PMID: 19176518 Free PMC article.
References
- Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–16. - PubMed
- Brodeur GM, Maris JM. Neuroblastoma. In: Pizzo PA, Poplack DG, editors. Principles and practice of pediatric oncology. 4. Philadelphia: J.B. Lippincott Company; 2002. pp. 895–938.
- DuBois SG, Kalika Y, Lukens JN, et al. Metastatic sites in stage IV and IVS neuroblastoma correlate with age, tumor biology, and survival. J Pediatr Hematol Oncol. 1999;21:181–9. - PubMed
- Norris MD, Bordow SB, Marshall GM, et al. Expression of the gene for multidrug-resistance-associated protein and outcome in patients with neuroblastoma. N Engl J Med. 1996;334:231–8. - PubMed
- Brodeur GM, Seeger RC, Schwab M, Varmus HE, Bishop JM. Amplification of N-myc sequences in primary human neuroblastomas: correlation with advanced disease stage. Prog Clin Biol Res. 1985;175:105–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA102321/CA/NCI NIH HHS/United States
- K08 NS053530/NS/NINDS NIH HHS/United States
- K02 NS002226-05/NS/NINDS NIH HHS/United States
- K02 NS002226-01/NS/NINDS NIH HHS/United States
- R01 CA102321-06/CA/NCI NIH HHS/United States
- K02 NS002226-04/NS/NINDS NIH HHS/United States
- K02 NS002226/NS/NINDS NIH HHS/United States
- K02 NS002226-03/NS/NINDS NIH HHS/United States
- R01 CA102321-07/CA/NCI NIH HHS/United States
- K08NS053530-01A2/NS/NINDS NIH HHS/United States
- R01 CA102321-03/CA/NCI NIH HHS/United States
- R01 CA102321-04A1/CA/NCI NIH HHS/United States
- R01 CA102321-05/CA/NCI NIH HHS/United States
- R01CAA102321/PHS HHS/United States
- K02 NS002226-02/NS/NINDS NIH HHS/United States
- R01 CA102321-08/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases