Ikaros is required for plasmacytoid dendritic cell differentiation - PubMed (original) (raw)
Ikaros is required for plasmacytoid dendritic cell differentiation
David Allman et al. Blood. 2006.
Abstract
Plasmacytoid dendritic cells (pDCs) are specialized DCs that produce high levels of type I IFN upon viral infection. Despite their key immunoregulatory role, little is known about pDC ontogeny or how developmental events regulate their function. We show that mice expressing low levels of the transcription factor Ikaros (Ik(L/L)) lack peripheral pDCs, but not other DC subsets. Loss of pDCs is associated with an inability to produce type I IFN after challenge with Toll-like receptor-7 and -9 ligands, or murine cytomegalovirus (MCMV) infection. In contrast, conventional DCs are present in normal numbers and exhibit normal responses in vivo after challenge with MCMV or inactivated toxoplasma antigen. Interestingly, Ik(L/L) bone marrow (BM) cells contain a pDC population that appears blocked at the Ly-49Q- stage of differentiation and fails to terminally differentiate in response to Flt-3L, a cytokine required for pDC differentiation. This differentiation block is strictly dependent on a cell-intrinsic requirement for Ikaros in pDC-committed precursors. Global gene expression profiling of Ik(L/L) BM pDCs reveals an up-regulation of genes not normally expressed, or expressed at low levels, in WT pDCs. These studies suggest that Ikaros controls pDC differentiation by silencing a large array of genes.
Figures
Figure 1.
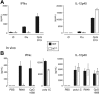
IkL/L mice show selective loss of pDCs. (A) Splenocytes from the indicated mice were stained with Alexa488 anti-120G8; PE anti-CD3, CD19, and NK1.1; APC anti-CD11c; PE-Cy7 anti-CD11b; APC-Cy5.5 anti-B220; and biotin anti-CD8α. Anti-CD8α antibodies were revealed with SA-PE-TexasRed. The resulting cells were analyzed on an LSR II flow cytometer by collecting 200 000 events for each file. Nonviable cells were eliminated from subsequent analyses by staining cells with DAPI. Numbers indicate percentage of events within the indicated gate as a function of the corresponding parent gate for each plot. The absolute number of events within each CD11c+ (CD3– CD19– NK1.1–) gate are as follows: WT, 889; Ik+/L, 795; and IkL/L, 1105. (B) Lymph node cells from the identical mice were stained and analyzed as in panel A. The absolute number of events within each CD11c+ (CD3– CD19– NK1.1–) gate are as follows: WT, 237; Ik+/L, 241; and IkL/L, 164. Absolute numbers of DCs within each subset in 3 mice per group are given in Table 1. These data are representative of 4 experiments.
Figure 2.
IkL/L mice do not produce IFNα upon stimulation with influenza virus or TLR ligands. (A) Purified CD11c+ spleen cells were stimulated with inactivated influenza virus (Flu) or CpG D19 for 24 hours, and supernatants were tested for the indicated cytokines. Results represent 1 of 2 experiments, on cells pooled from 5 mice/genotype per experiment. (B) Mice were injected with poly I:C, R848, or CpG 1668. Sera were collected at 2 hours (R848) and 4 hours (PBS, poly I:C, CpG) after injection and tested for the indicated cytokines. Results are representative of 2 experiments, with 3 mice/genotype per condition for each experiment. Means and SDs are shown for 1 representative experiment.
Figure 3.
Antiviral response to MCMV infection. (A) Animals were injected intraperitoneally with GFP-recombinant MCMV. At 1.5 days after challenge, the frequencies of pDCs and MCMV-infected DCs were measured in the spleen, and cytokines were titrated in the serum. *P = .005; **P = .001. (B) Distribution of pDCs (Ly-6C+ 120G8+) in WT and IkL/L spleens following infection. Contour plots are gated on CD11c+ cells. Numbers indicate percentage of gated cells in each plot. (C) Proportion of GFP+ WT and IkL/L spleen cells after infection. Results shown are representative of 5 experiments, each with 3 to 12 mice per group. Means and SDs are shown for 1 representative experiment.
Figure 4.
A pDC population in the BM of Ik L/L mice. (A) CD11c+ BM cells were gated and analyzed for B220 and 120G8 expression. Numbers indicate percentage of cells in the gated contour plots. (B) 120G8+ spleen and BM cells were analyzed for their expression of the indicated surface antigens. The pDC population is indicated by the white histograms. Gray histograms indicate the isotype controls. Results are representative of 3 experiments. (C) Sorted CD11c+ 120G8+ BM cells were cultured with poly I:C, inactivated influenza virus, or CpG D19 for 24 hours, and supernatants were tested for the indicated cytokines. Results are representative of 2 experiments, on pools of cells from 5 mice/genotype per experiment. Means and SDs are shown for 1 representative experiment.
Figure 5.
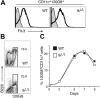
IkL/L pDC precursors respond to Flt-3 signaling. (A) 120G8+ BM cells were analyzed for Flt-3 expression. The pDC population is indicated by the white histograms. Gray histograms indicate the isotype controls. Results are representative of 5 experiments. (B-C) Unfractionated BM cells were cultured with Flt-3L over a 9-day period. (B) Contour plots show B220 and 120G8 expression of CD11c+ cells after 7 days of culture. (C) Graph shows the percentage of 120G8+ cells among total cells for the indicated number of days. One representative experiment of 5 is shown.
Figure 6.
The defect in pDC differentiation is cell intrinsic. (A) BM from WT (Ly5B6; 2 × 106), IkL/L (Ly5B6; 5 × 106), or mixtures of WT:B6.Ly5SJL (1 × 106: 1 × 106) or IkL/L:B6.Ly5SJL (5 × 106:1 × 106) were used to reconstitute lethally-irradiated B6.Ly5SJL recipients. Recipients were analyzed 6 to 10 weeks after BM transfer. All plots are gated on small Ly5B6+CD11c-enriched cells. Numbers indicate the percentage of gated cells in each plot. One representative experiment of 4 is shown. (B) Ikaros expression in DC subsets. WT BM and splenic pDCs (CD11c+ B220+ 120G8+), and splenic CD11b+ cDCs (CD11c+ CD11b+120G8–) and CD8α+ cDCs (CD11c+CD8α+CD11b–120G8–) were analyzed by RT-PCR for their expression of Ikaros, Aiolos, and Helios. Whole WT thymocytes were used as a control. One representative experiment of 3 is shown.
Figure 7.
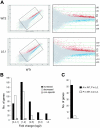
Significant gene derepression in IkL/L BM pDCs. (A) Representative scatterplots comparing the gene expression profile between 2 independent WT samples (top), versus a WT and an IkL/L sample (bottom). The right panels are enlargements of boxed areas in the left panels. Red indicates increased genes; blue, decreased genes. (B) Genes that either “increased” or “decreased” in all of the 9 IkL/L/WT comparisons were selected and grouped according to the magnitude of change (average of the “fold change” [in log2] from all 9 comparisons). Note that the decreased genes exhibited mostly modest variations, while many increased genes exhibited large variations.  correspond to the estimated number of nonspecific changes (“Materials and methods”). (C) Number of up-regulated genes in IkL/L samples whose expression was not detected in WT samples (▪) or were expressed in WT but not detected in IkL/L samples (□).
correspond to the estimated number of nonspecific changes (“Materials and methods”). (C) Number of up-regulated genes in IkL/L samples whose expression was not detected in WT samples (▪) or were expressed in WT but not detected in IkL/L samples (□).
Similar articles
- Molecular dissection of plasmacytoid dendritic cell activation in vivo during a viral infection.
Tomasello E, Naciri K, Chelbi R, Bessou G, Fries A, Gressier E, Abbas A, Pollet E, Pierre P, Lawrence T, Vu Manh TP, Dalod M. Tomasello E, et al. EMBO J. 2018 Oct 1;37(19):e98836. doi: 10.15252/embj.201798836. Epub 2018 Aug 21. EMBO J. 2018. PMID: 30131424 Free PMC article. - DAP12 signaling regulates plasmacytoid dendritic cell homeostasis and down-modulates their function during viral infection.
Sjölin H, Robbins SH, Bessou G, Hidmark A, Tomasello E, Johansson M, Hall H, Charifi F, Karlsson Hedestam GB, Biron CA, Kärre K, Höglund P, Vivier E, Dalod M. Sjölin H, et al. J Immunol. 2006 Sep 1;177(5):2908-16. doi: 10.4049/jimmunol.177.5.2908. J Immunol. 2006. PMID: 16920926 - Cutting edge: Overlapping functions of TLR7 and TLR9 for innate defense against a herpesvirus infection.
Zucchini N, Bessou G, Traub S, Robbins SH, Uematsu S, Akira S, Alexopoulou L, Dalod M. Zucchini N, et al. J Immunol. 2008 May 1;180(9):5799-803. doi: 10.4049/jimmunol.180.9.5799. J Immunol. 2008. PMID: 18424698 - IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors.
Liu YJ. Liu YJ. Annu Rev Immunol. 2005;23:275-306. doi: 10.1146/annurev.immunol.23.021704.115633. Annu Rev Immunol. 2005. PMID: 15771572 Review. - From plasmacytoid to dendritic cell: morphological and functional switches during plasmacytoid pre-dendritic cell differentiation.
Soumelis V, Liu YJ. Soumelis V, et al. Eur J Immunol. 2006 Sep;36(9):2286-92. doi: 10.1002/eji.200636026. Eur J Immunol. 2006. PMID: 16892183 Review.
Cited by
- Dendritic cells in inborn errors of immunity.
Gupta S, Agrawal A. Gupta S, et al. Front Immunol. 2023 Jan 23;14:1080129. doi: 10.3389/fimmu.2023.1080129. eCollection 2023. Front Immunol. 2023. PMID: 36756122 Free PMC article. Review. - The multifaceted biology of plasmacytoid dendritic cells.
Swiecki M, Colonna M. Swiecki M, et al. Nat Rev Immunol. 2015 Aug;15(8):471-85. doi: 10.1038/nri3865. Epub 2015 Jul 10. Nat Rev Immunol. 2015. PMID: 26160613 Free PMC article. Review. - Transcriptional regulation of dendritic cell diversity.
Chopin M, Allan RS, Belz GT. Chopin M, et al. Front Immunol. 2012 Feb 27;3:26. doi: 10.3389/fimmu.2012.00026. eCollection 2012. Front Immunol. 2012. PMID: 22566910 Free PMC article. - Clonal lineage tracing reveals shared origin of conventional and plasmacytoid dendritic cells.
Feng J, Pucella JN, Jang G, Alcántara-Hernández M, Upadhaya S, Adams NM, Khodadadi-Jamayran A, Lau CM, Stoeckius M, Hao S, Smibert P, Tsirigos A, Idoyaga J, Reizis B. Feng J, et al. Immunity. 2022 Mar 8;55(3):405-422.e11. doi: 10.1016/j.immuni.2022.01.016. Epub 2022 Feb 17. Immunity. 2022. PMID: 35180378 Free PMC article. - Whole-genome analysis uncovers recurrent IKZF1 inactivation and aberrant cell adhesion in blastic plasmacytoid dendritic cell neoplasm.
Bastidas Torres AN, Cats D, Mei H, Fanoni D, Gliozzo J, Corti L, Paulli M, Vermeer MH, Willemze R, Berti E, Tensen CP. Bastidas Torres AN, et al. Genes Chromosomes Cancer. 2020 May;59(5):295-308. doi: 10.1002/gcc.22831. Epub 2019 Dec 31. Genes Chromosomes Cancer. 2020. PMID: 31846142 Free PMC article.
References
- Asselin-Paturel C, Boonstra A, Dalod M, et al. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat Immunol. 2001;2: 1144-1150. - PubMed
- Siegal FP, Kadowaki N, Shodell M, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284: 1835-1837. - PubMed
- Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23: 275-306. - PubMed
- Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5: 1219-1226. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI052861/AI/NIAID NIH HHS/United States
- R01 AI058066/AI/NIAID NIH HHS/United States
- AI55677/AI/NIAID NIH HHS/United States
- AI58066/AI/NIAID NIH HHS/United States
- R56 AI055677/AI/NIAID NIH HHS/United States
- R01 AI055677/AI/NIAID NIH HHS/United States
- AI52861/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases