Multiprotein complexes of the survival of motor neuron protein SMN with Gemins traffic to neuronal processes and growth cones of motor neurons - PubMed (original) (raw)
Multiprotein complexes of the survival of motor neuron protein SMN with Gemins traffic to neuronal processes and growth cones of motor neurons
Honglai Zhang et al. J Neurosci. 2006.
Abstract
Spinal muscular atrophy (SMA), a progressive neurodegenerative disease affecting motor neurons, is caused by mutations or deletions of the SMN1 gene encoding the survival of motor neuron (SMN) protein. In immortalized non-neuronal cell lines, SMN has been shown to form a ribonucleoprotein (RNP) complex with Gemin proteins, which is essential for the assembly of small nuclear RNPs (snRNPs). An additional function of SMN in neurons has been hypothesized to facilitate assembly of localized messenger RNP complexes. We have shown that SMN is localized in granules that are actively transported into neuronal processes and growth cones. In cultured motor neurons, SMN granules colocalized with ribonucleoprotein Gemin proteins but not spliceosomal Sm proteins needed for snRNP assembly. Quantitative analysis of endogenous protein colocalization in growth cones after three-dimensional reconstructions revealed a statistically nonrandom association of SMN with Gemin2 (40%) and Gemin3 (48%). SMN and Gemin containing granules distributed to both axons and dendrites of differentiated motor neurons. A direct interaction between SMN and Gemin2 within single granules was indicated by fluorescence resonance energy transfer analysis of fluorescently tagged and overexpressed proteins. High-speed dual-channel imaging of live neurons depicted the rapid and bidirectional transport of the SMN-Gemin complex. The N terminus of SMN was required for the recruitment of Gemin2 into cytoplasmic granules and enhanced Gemin2 stability. These findings provide new insight into the molecular composition of distinct SMN multiprotein complexes in neurons and motivation to investigate deficiencies of localized RNPs in SMA.
Figures
Figure 1.
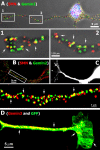
Colocalization of endogenous SMN and Gemin proteins in neurites and growth cones in primary forebrain culture and ES cell-derived motor neurons. A, SMN (red) and Gemin2 (green) in cultured forebrain neurons (3 DIV) were detected by double-labeled immunofluorescence using a monoclonal antibody to SMN and polyclonal antibody to Gemin2. The nucleus was stained with DAPI (blue). Higher magnification of two regions (insets 1, 2 from top panel are enlarged in bottom panel) depicts the frequent colocalization between SMN and Gemin2 within granules in the growth cone (1, arrows) and neurite (2, arrows). B, Double-labeled IF showing colocalization of SMN (red) and Gemin2 (Cy5 antibody displayed in green) in neurites of ES cell-derived differentiated motoneuron. Higher magnification of a boxed region depicts numerous granules with colocalization between SMN and Gemin2 (bottom panel, arrows). C, These cells express EGFP from a motor neuron-specific promoter. D, IF detection of Gemin3 with a monoclonal antibody depicts many granules localized to the EGFP-positive axon and growth cone of the motor neuron (arrows).
Figure 2.
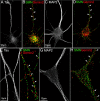
Colocalization of endogenous SMN and Gemin2 in axons and dendrites of differentiated cultures of primary hippocampal and motor neurons. Primary cultures of rat hippocampal neurons (A–D) and embryonic mouse motor neurons (E–H) were fixed with 4% paraformaldehyde at 7 DIV and processed for triple-labeled IF to detect the colocalization of endogenous SMN and Gemin2 in both axons and dendrites. Monoclonal antibodies to SMN and Gemin2 were directly conjugated with Alexa Fluor 647 and Alexa Fluor 568, respectively (see Materials and Methods). Axons and dendrites were discriminated by IF staining with a polyclonal antibody to tau (A, E) or MAP2 (C, G) and Cy2-conjugated secondary antibody. The colocalization (indicated by arrows) of SMN (green) and Gemin2 (red) in both axons (B, F) and dendrites (D, H) of hippocampal neurons and mouse motor neurons was observed.
Figure 3.
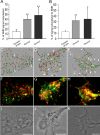
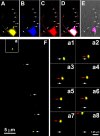
Quantitative analysis of SMN–Gemin colocalization in growth cones after 3D reconstruction. Hippocampal neurons were cultured for 4 DIV and processed for double-labeled immunofluorescence using directly conjugated monoclonal antibodies. Growth cones (n = 10 per color pair) were imaged in three dimensions, deconvolved, thresholded, and statistically analyzed for percentage of voxels with colocalized signals above threshold (Imaris). A, The SMN signal colocalized to Gemin2 (40.0 ± 7.0%) and Gemin3 (47.8 ± 15.8%) was 1.7 and 2.2 times higher than the signal of SMN colocalized to synaptophysin (15.0 ± 4.5%), which was equally abundant within growth cones. B, The Gemin2 signal (32.3 ± 7.2%) was 1.6 times higher than the synaptophysin signal (12.3 ± 2.6%) colocalized to SMN, and Gemin3 signal (34.7 ± 18.8%) colocalized to SMN was 1.8 times higher than synaptophysin (12.3 ± 2.6%). *p < 0.01; **p < 0.001. Colocalization of SMN (green) and synaptophysin, Gemin2, or Gemin3 (red) were represented by deconvolved and reconstructed images shown in C, D, and E, respectively. Colocalized voxels (white) are indicated by white arrows. Non-colocalized signals are indicated by black arrows (SMN) or yellow arrows (Gemin2 and Gemin3). The raw unprocessed data for these three growth cones are shown in F–H. Corresponding phase images are shown in I–K. The presence of SMN–Gemin granules in filopodial protrusions from the growth cone is noted in boxed regions in D and E. Scale bars, 5 μm.
Figure 4.
SMN granules within neurites are deficient of Sm proteins in motor neurons. Primary cultured mouse motor neurons were double immunostained for SMN (A, in green) and Sm proteins (B, in red), a major component of snRNPs. Images were deconvolved, superimposed, and registered using fiduciary beads (arrows) in the mounting medium. A, SMN was prevalent in the nucleus and was distributed throughout the cytoplasm and neurites within granules. B, Sm proteins were highly enriched in the nucleus and showed significant staining only within the perinuclear cytoplasm, in which there was colocalization with SMN (C, merged colors, inset a enlarged in bottom row). C, In the neurite, a very low frequency of colocalized signal (in yellow) appeared. Sm protein levels in the middle neurite segment (see enlarged inset b in bottom row) were markedly reduced compared with SMN. In the distal neurite segment, the amount of Sm proteins was extremely low with respect to SMN (enlarged inset c).
Figure 5.
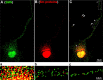
Colocalization and cotransport of fluorescently tagged and overexpressed SMN and Gemin2. Cultured forebrain neurons were cotransfected with EYFP-SMN (A, yellow) and ECFP–Gemin2 (B, blue) and then fixed for immunofluorescence detection of endogenous Gemin3 (C, red) using a monoclonal antibody. Colocalization of EYFP-SMN, ECFP–Gemin2, and Gemin3 within eight discrete granules is shown in neurites (D, arrows, merged signals). E, The nucleus was stained with DAPI (pink) and overlaid with differential interference contrast optics. F, In another experiment, neurons were cotransfected with EYFP–SMN (red) and ECFP–Gemin2 (green) and imaged in live cells using high-speed, dual-channel imaging. Five granules, containing both molecules, are depicted along the length of a neurite (arrows). One of these granules (a) is tracked for eight frames and displays an anterograde trajectory (white arrow denotes starting point, red arrows show position during subsequent frames, a1–a8).
Figure 6.
FRET analysis depicts interaction between EYFP–SMN and ECFP–Gemin2 or ECFP–Gemin3 in neurites. FRET was used to detect protein–protein interactions between SMN and Gemins in neurites. Cultured forebrain neurons were transfected with ECFP–Gemin2 or ECFP–Gemin3 as the donor and EYFP–SMN as the acceptor fluorophore, for FRET analysis. Fluorescence was imaged using a confocal microscope (see Materials and Methods). One neuron is shown here as an example. A, B, ECFP–Gemin2 granules (A, blue) and EYFP–SMN granules (B, yellow) before photobleaching of EYFP. C, ECFP–Gemin2 fluorescence after photobleaching of boxed region (ROI 1). Each granule within ROI 1 was outlined in red and indicated as ROI2–ROI 15. An area (ROI 16) outside the neurite was also circled within ROI 1 as a control for measurement of increased fluorescence in ECFP channel after photobleaching of EYFP. Three granules, labeled as ROI 17–ROI 19, in another neurite that was not photobleached, were used as additional controls for FRET measurements. D, Fluorescence signals of EYFP–SMN granules were eliminated by photobleaching with a laser in the selected area (ROI 1). E, FRET measurements are indicated in the table. FRET frequency indicates the percentage of granules showing increased ECFP fluorescence after photobleaching of EYFP. FRET average indicates the percentage increase in ECFP fluorescence after EYFP photobleaching.
Figure 7.
Gemin2 is recruited by SMN into granules within neurites and growth cones. A, After transfection of EGFP–Gemin2 (green), cultured forebrain neurons (4 DIV) were fixed for IF analysis of endogenous SMN (red), and the nucleus was stained with DAPI (blue). The EGFP–Gemin2 fluorescence was mostly diffuse and filled all cellular regions including cytoplasmic processes and the nucleus. Note that the blue stain of the nucleus was not visible in the merged overlay because of the strong EGFP signal. The distribution of SMN (red) was highly granular or punctate as described previously (Zhang et al., 2003). One neurite from the boxed inset is enlarged at right to show EGFP–Gemin2 (top), SMN (middle), and overlay (bottom). B, In contrast, when EGFP–Gemin2 was cotransfected with Flag–SMN (red), the EGFP–Gemin2 signal was then granular and frequently colocalized with SMN (red) in neurites, growth cones, and filopodia (arrows). One neurite (boxed inset) is enlarged to the right and shows EGFP–Gemin2 (top), Flag–SMN (middle), and overlay (bottom). Note also that the DAPI-stained nucleus (blue) is now visible, because the EGFP–Gemin2 signal is observed in puncta (likely gems) in contrast to filling the entire nucleus as in A.
Figure 8.
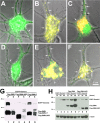
Interactions between Gemin2 with SMN necessary for recruitment into granules and to stabilize Gemin2. A, D, Overexpression of ECFP–Gemin2 (A) or ECFP–Gemin3 (D) by themselves in cultured forebrain neurons (4 DIV) showed diffuse and uniform fluorescence, with no evidence for discrete granules (green, arrows). B, E, In contrast, when ECFP–Gemin2 (B, green) or ECFP–Gemin3 (E, green) were cotransfected with EYFP–SMN (red), the recruitment of Gemins into SMN granules was observed (B, E, merged images, arrows). C, In contrast, coexpression of the N-terminal deletion mutant of SMN (EYFP–SMNΔN53), which lacks the known binding site for Gemin2, was not able to recruit Gemin2 into granules. EYFP–SMNΔN53 (red) showed a granular pattern in neurites (C, arrows), yet ECFP–Gemin2 (C, green) remained in a diffuse pattern. The red and green signal in the neurite showed little colocalization. F, ECFP–Gemin3 (green) was recruited into granules by cotransfection with EYFP–SMNΔN53 (red), as evident by colocalization of the merged images (yellow, arrows). G, Gemin2 stabilization by interactions with SMN. HEK293 cells were cotransfected with EGFP–Gemin2 and Flag-tagged SMN constructs. P, Pellet; S Supernatant. Cells were lysed, immunoprecipitated with anti-Flag antibodies, and analyzed for EGFP and Flag expression by Western blot. EGFP–Gemin2 was coimmunoprecipitated with Flag–SMN (lane 1) or Flag–SMNΔ7 (lane 5) but not Flag–SMNΔN53 (lane 3), which lacks the known Gemin2 binding domain. H, Western blot analysis at 12, 24, and 48 h expression showed that EGFP–Gemin2 was present at higher levels over time when coexpressed with Flag–SMN (lanes 4–6) compared with when Gemin2 was expressed by itself (lanes 1–3) or coexpressed with Flag–SMNΔN53 (lanes 7–9). Flag- and EGFP-tagged proteins were detected with monoclonal antibodies. β-Actin was detected with a monoclonal antibody as loading control.
Similar articles
- Active transport of the survival motor neuron protein and the role of exon-7 in cytoplasmic localization.
Zhang HL, Pan F, Hong D, Shenoy SM, Singer RH, Bassell GJ. Zhang HL, et al. J Neurosci. 2003 Jul 23;23(16):6627-37. doi: 10.1523/JNEUROSCI.23-16-06627.2003. J Neurosci. 2003. PMID: 12878704 Free PMC article. - A role for complexes of survival of motor neurons (SMN) protein with gemins and profilin in neurite-like cytoplasmic extensions of cultured nerve cells.
Sharma A, Lambrechts A, Hao le T, Le TT, Sewry CA, Ampe C, Burghes AH, Morris GE. Sharma A, et al. Exp Cell Res. 2005 Sep 10;309(1):185-97. doi: 10.1016/j.yexcr.2005.05.014. Exp Cell Res. 2005. PMID: 15975577 - QNQKE targeting motif for the SMN-Gemin multiprotein complexin neurons.
Zhang H, Xing L, Singer RH, Bassell GJ. Zhang H, et al. J Neurosci Res. 2007 Sep;85(12):2657-67. doi: 10.1002/jnr.21308. J Neurosci Res. 2007. PMID: 17455327 - The SMN complex.
Gubitz AK, Feng W, Dreyfuss G. Gubitz AK, et al. Exp Cell Res. 2004 May 15;296(1):51-6. doi: 10.1016/j.yexcr.2004.03.022. Exp Cell Res. 2004. PMID: 15120993 Review. - The SMN complex: an assembly machine for RNPs.
Battle DJ, Kasim M, Yong J, Lotti F, Lau CK, Mouaikel J, Zhang Z, Han K, Wan L, Dreyfuss G. Battle DJ, et al. Cold Spring Harb Symp Quant Biol. 2006;71:313-20. doi: 10.1101/sqb.2006.71.001. Cold Spring Harb Symp Quant Biol. 2006. PMID: 17381311 Review.
Cited by
- Ptbp2 re-expression rescues axon growth defects in Smn-deficient motoneurons.
Salehi S, Zare A, Gandhi G, Sendtner M, Briese M. Salehi S, et al. Front Mol Neurosci. 2024 Aug 23;17:1393779. doi: 10.3389/fnmol.2024.1393779. eCollection 2024. Front Mol Neurosci. 2024. PMID: 39246602 Free PMC article. - Ddx20, an Olig2 binding factor, governs the survival of neural and oligodendrocyte progenitor cells via proper Mdm2 splicing and p53 suppression.
Bizen N, Bepari AK, Zhou L, Abe M, Sakimura K, Ono K, Takebayashi H. Bizen N, et al. Cell Death Differ. 2022 May;29(5):1028-1041. doi: 10.1038/s41418-021-00915-8. Epub 2022 Jan 1. Cell Death Differ. 2022. PMID: 34974536 Free PMC article. - A SMN missense mutation complements SMN2 restoring snRNPs and rescuing SMA mice.
Workman E, Saieva L, Carrel TL, Crawford TO, Liu D, Lutz C, Beattie CE, Pellizzoni L, Burghes AH. Workman E, et al. Hum Mol Genet. 2009 Jun 15;18(12):2215-29. doi: 10.1093/hmg/ddp157. Epub 2009 Mar 27. Hum Mol Genet. 2009. PMID: 19329542 Free PMC article. - Chaperoning ribonucleoprotein biogenesis in health and disease.
Pellizzoni L. Pellizzoni L. EMBO Rep. 2007 Apr;8(4):340-5. doi: 10.1038/sj.embor.7400941. EMBO Rep. 2007. PMID: 17401408 Free PMC article. Review. - Specific splicing defects in S. pombe carrying a degron allele of the Survival of Motor Neuron gene.
Campion Y, Neel H, Gostan T, Soret J, Bordonné R. Campion Y, et al. EMBO J. 2010 Jun 2;29(11):1817-29. doi: 10.1038/emboj.2010.70. Epub 2010 Apr 16. EMBO J. 2010. PMID: 20400941 Free PMC article.
References
- Arce V, Garces A, de Bovis B, Filippi P, Henderson C, Pettmann B, deLapeyriere O (1999). Cardiotrophin-1 requires LIFRbeta to promote survival of mouse motoneurons purified by a novel technique. J Neurosci Res 55:119–126. - PubMed
- Bannai H, Fukatsu K, Mizutani A, Natsume T, Iemura S, Ikegami T, Inoue T, Mikoshiba K (2004). An RNA-interacting protein, SYNCRIP (heterogeneous nuclear ribonuclear protein Q1/NSAP1) is a component of mRNA granule transported with inositol 1,4,5-trisphosphate receptor type 1 mRNA in neuronal dendrites. J Biol Chem 279:53427–53434. - PubMed
- Battaglia G, Princivalle A, Forti F, Lizier C, Zeviani M (1997). Expression of the SMN gene, the spinal muscular atrophy determining gene, in the mammalian central nervous system. Hum Mol Genet 6:1961–1971. - PubMed
- Bechade C, Rostaing P, Cisterni C, Kalisch R, La Bella V, Pettmann B, Triller A (1999). Subcellular distribution of survival motor neuron (SMN) protein: possible involvement in nucleocytoplasmic and dendritic transport. Eur J Neurosci 11:293–304. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases